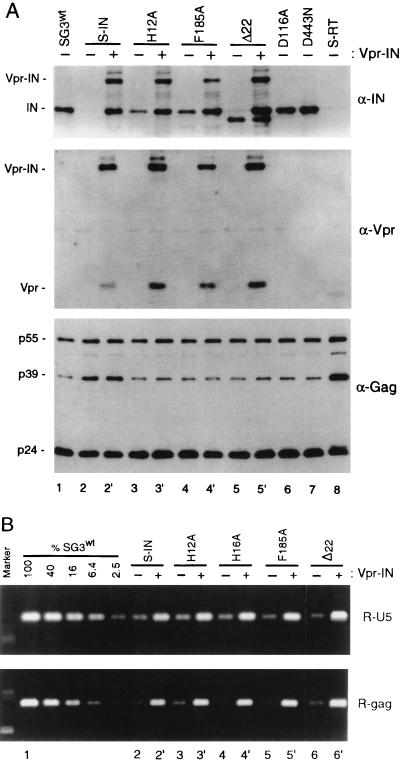
FIG. 2.
Analysis of Vpr-IN-complemented virions. Four micrograms of the wild-type and mutant proviral DNA clones was individually transfected (−) into 293T cells and cotransfected (+) with the pLR2P-vprIN expression plasmid. Forty-eight hours later, the culture supernatants were collected, passed through 0.45-μm-pore-size filters, and analyzed for HIV-1 p24 antigen concentration by ELISA. (A) Immunoblot analysis. One-half of the filtered supernatant was centrifuged (125,000 × g for 2 h) over cushions of 20% sucrose. The pellets were lysed and examined by immunoblot analysis with anti-IN (α-IN) (top), anti-Vpr (middle), and anti-Gag (bottom) antibodies as described earlier (57). Vpr-IN-containing H16A virions were identical to the H12A virions (data not shown). (B) The trans-IN protein rescues viral DNA synthesis. Five hundred nanograms of wild-type virus and each of the mutant viruses was used to infect cultures of HeLa-CD4 cells. After 4 h, the cell monolayers were washed, trypsinized, resuspended in fetal bovine serum, and divided into two aliquots. One aliquot set was analyzed by p24 antigen ELISA as described for Fig. 1. The other aliquot set was placed back in culture medium at 37°C. At 18 h postinfection, the cells were washed and total DNA was extracted by organic methods. The extracts were normalized for intracellular CA protein concentration and analyzed by PCR for viral DNA products of reverse transcription as described for Fig. 1. The data are from a representative experiment that was repeated three times, each time with independent virus preparations.