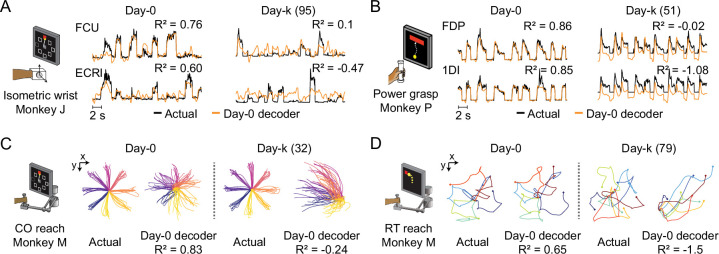
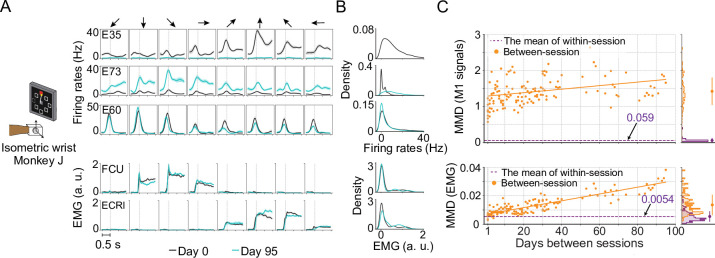
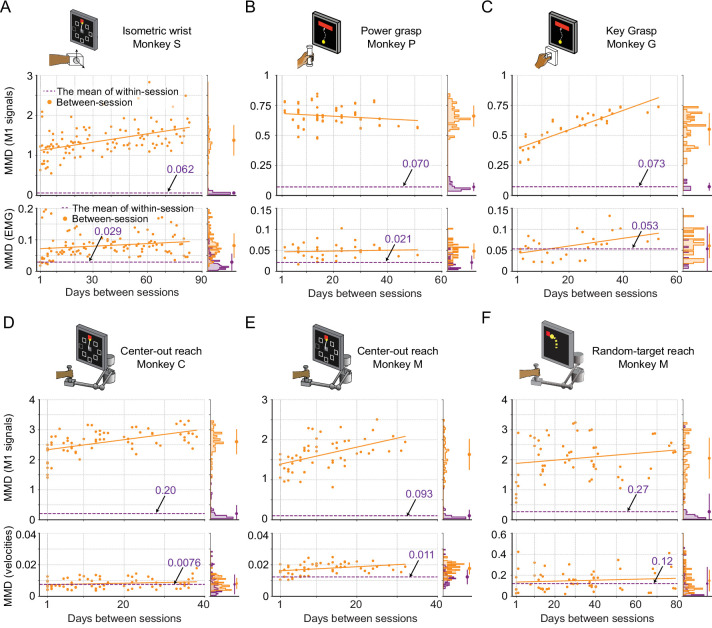
Data from monkey J, who was trained to perform the isometric wrist task. (A) Peri-event time histograms (PETHs) for the multiunit activity from three cortical electrodes (E35, E73, E60) and the EMGs from two forearm muscles (flexor carpi ulnaris, FCU; extensor carpi radialis longus, ECRl) on day 0 and day 95. Each column corresponds to a target direction indicated by the arrows on the top. For each direction, 15 trials were averaged to get the mean values (solid lines) and the standard errors (shaded area). The dashed vertical line in each subplot indicates the timing of force onset. While the neural activity picked by the implanted electrodes may change dramatically (E35, E73) or remain largely consistent (E95), the EMG patterns from two muscles which are critical to the task remain stable. (B) The distributions of the neural firing rates from E35, E73 and E60 and the EMGs from FCU and ECRl. The order of the subplots is consistent with (A). Note that for E35 the distribution of day-95 neural firing rates was omitted, since all values are close to 0. (C) The within-session and between-session maximum mean discrepancy (MMD) values for M1 signals (top panel) and EMGs (bottom panel). MMD provides a measure of distance between two multivariate distributions, and was used here to quantify the similarity of the distributions of neural activity or motor outputs between pairs of separate recording sessions in the dataset. In each panel the solid orange line shows a linear fit for all between-session MMDs, the dashed purple line indicates the mean of all within-session MMDs. The histograms for within-session and between-session MMDs are plotted on the right side of each panel, and the mean (solid dots) and standard deviation (solid lines) are shown. The between-session MMDs for M1 signals were an order of magnitude larger than for EMGs, and at least 10 times larger than the corresponding within-session values, indicating that instabilities in neural recordings are greater than in the motor output (note that the monkey was already well trained and proficient with the tasks before the data collection process began). However, factors such as monkey’s daily condition, noise levels of recordings, and drifts of the sensors on the behavioral apparatus could have altered the measured motor outputs across time and led to the reported gradual increase of the between-session MMDs for EMGs.