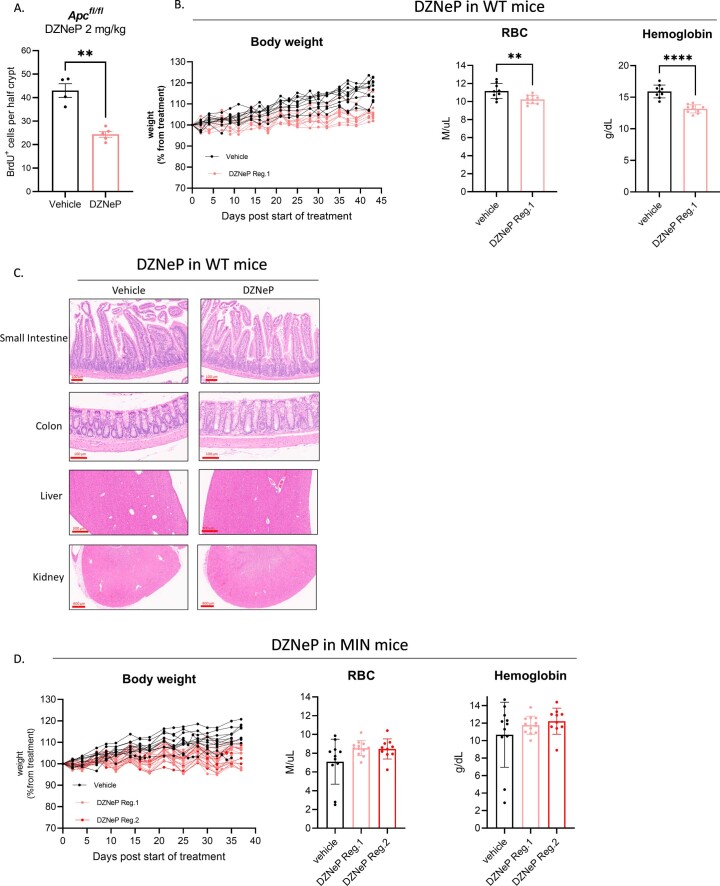
Extended Data Fig. 10. Evaluation of long-term DZNeP treatment in WT and MIN mice.
(A) Quantification of IHC for BrdU in the small intestine of APC mice treated with vehicle (n = 4) or DZNeP (2 mg/kg; n = 5) for 4 days (Mean ± SEM). Each dot represents the average number of BrdU positive cells per half crypt for each mouse. Asterisk refers to p-value obtained from 1-tailed Mann-Whitney test (**: p = 0.0079). (B-C) Body weight trajectories, blood analysis (Mean ± SD) and representative images of organs from WT mice treated with vehicle or DZNeP from day 50 until day 93 of age. Data show that body weights of DZNeP-treated mice were stable, although lower than vehicle-treated animals at the end of the experiment. Blood profiling showed a decrease in red blood cells and haemoglobin in DZNeP-treated mice, but no intestinal bleeding or clinical signs of anaemia were observed. Histological analysis of small intestine, colon, liver and kidneys did not reveal any abnormalities associated with DZNeP treatment. Vehicle (n = 10 for body weight; n = 9 for blood analysis): i.p. PBS. DZNeP Regime 1 (n = 10): 2 mg/kg i.p. using weekly cycles of 4 days of daily treatment followed by 3 days of no treatment. Each dot represents an individual mouse, asterisks represent p-values obtained from 1-tailed Mann-Whitney test (**: p = 0.0076; ****: p < 0.0001). (D) Body weight trajectories and blood analysis (Mean ± SD) from ApcMin/+ mice treated with vehicle or DZNeP from day 50 until day 85 of age. Body weights remained stable during treatment, and DZNeP did not result in more severe disease-related anaemia. Vehicle (n = 11): i.p. PBS. Regime 1: DZNeP (2 mg/kg i.p.; n = 12) using weekly cycles of 4 days of daily treatment followed by 3 days of no treatment. Regime 2: DZNeP (5 mg/kg i.p.; n = 10) twice per week. Each dot represents an individual mouse.