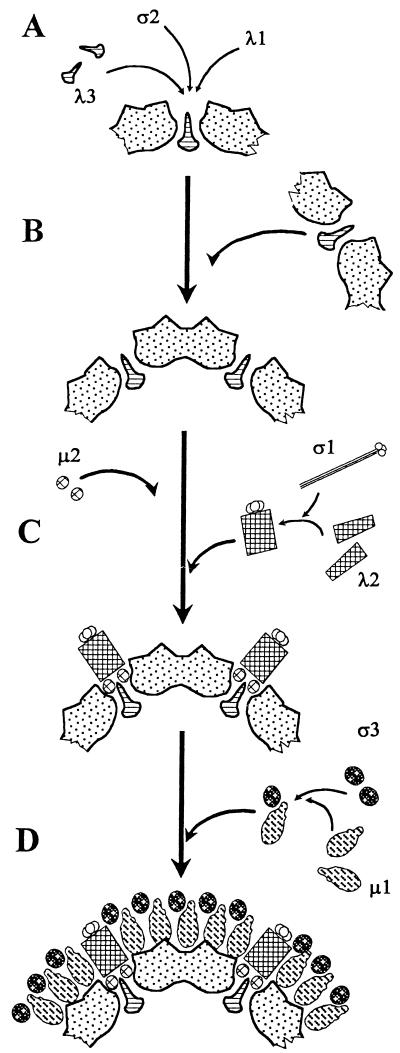
FIG. 3.
The proposed model for the assembly of reovirus. (A) Assembly of the dodecahedral base unit. Five dimers of the core protein λ1, ten units of ς2, and one copy of λ3 associate to form the dodecahedral base structure. (B) Assembly of the primary core particle. Twelve dodecahedral base units assemble to form the primary capsid structure. (C) Assembly of the intermediate particle. Two copies of μ2 associate with each apex of the primary core particles immediately prior to or collateral with assembly of the twelve λ2 pentameric spikes. The trimeric ς1 attachment protein is most likely coassembled with the λ2 spike, with the amino termini of the ς1 fibers interacting with the carboxy termini of the λ2 spike proteins. (D) The outer capsid proteins ς3 and μ1 associate with each other and then condense around the λ2 pentameric spike to form the complete virion.