Figure 2.
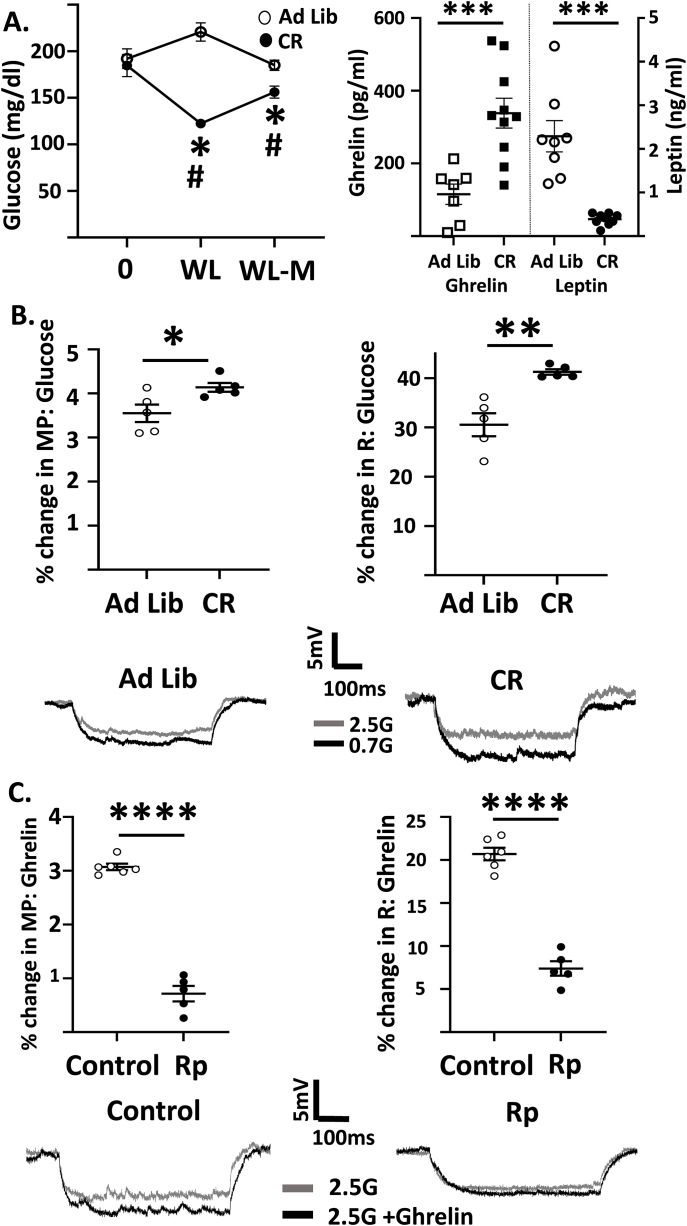
Calorie restriction decreases glucose inhibition of hcrt/ox-GI neurons due, in part, to ghrelin action on PKA. A. Blood glucose levels (left) were measured in a subset of ad lib fed and calorie restricted (CR) mice at the start of the study (time 0), upon reaching ∼15% weight loss (WL) and after 1-week WL-maintenance (WL-M). Plasma glucose levels were lower during WL and WL-M (Repeated measures 2-way ANOVA showed an interaction between diet status and time (P < 0.0001, F (2,36) = 17.26); Sisak's multiple comparison ∗P < 0.05 CR compared to time 0 within their group, #P < 0.01 CR compared to the same time point for the ad lib fed group (n = 10/group). Plasma ghrelin levels (right) were significantly higher in these CR mice at the end of WL-M (Independent Student's t-test ∗∗∗P < 0.001, t = 4.074, df = 15, n = 7 ad lib, n = 10 CR) and plasma leptin levels (far right) were significantly lower (Independent Student's t-test ∗∗∗P < 0.0001, t = 5.59, df = 15, n = 8 ad lib, n = 9. CR). B. Top: Percent change in MP and R of hcrt/ox-GI neurons in response to decreased glucose from 2.5 to 0.7 in ad lib fed and CR mice (Independent Student's t-test, ∗P < 0.05, ∗∗P < 0.01, ad lib n/N = 6/5, CR n/N = 5/5). Bottom: Representative voltage responses of hcrt/ox-GI neurons to decreased glucose in ad lib and CR mice (gray: 2.5, black 0.1 mM glucose). C. Top: Percent change in MP and R of hcrt/ox neurons in response to the addition of ghrelin (10 nM) to a constant glucose level of 2.5 mM in the presence and absence of the PKA inhibitor Rp-cAMP (10 μM Rp, independent Student's t-test, ∗∗∗∗P < 0.0001, control n/N = 5/5, Rp-cAMP n/N = 6/5). Bottom: Representative voltage responses to ghrelin addition to 2.5 mM glucose in control and Rp-cAMP (gray: 2.5, black 0.1 mM glucose). Data represented as mean ± SEM.