FIG 1.
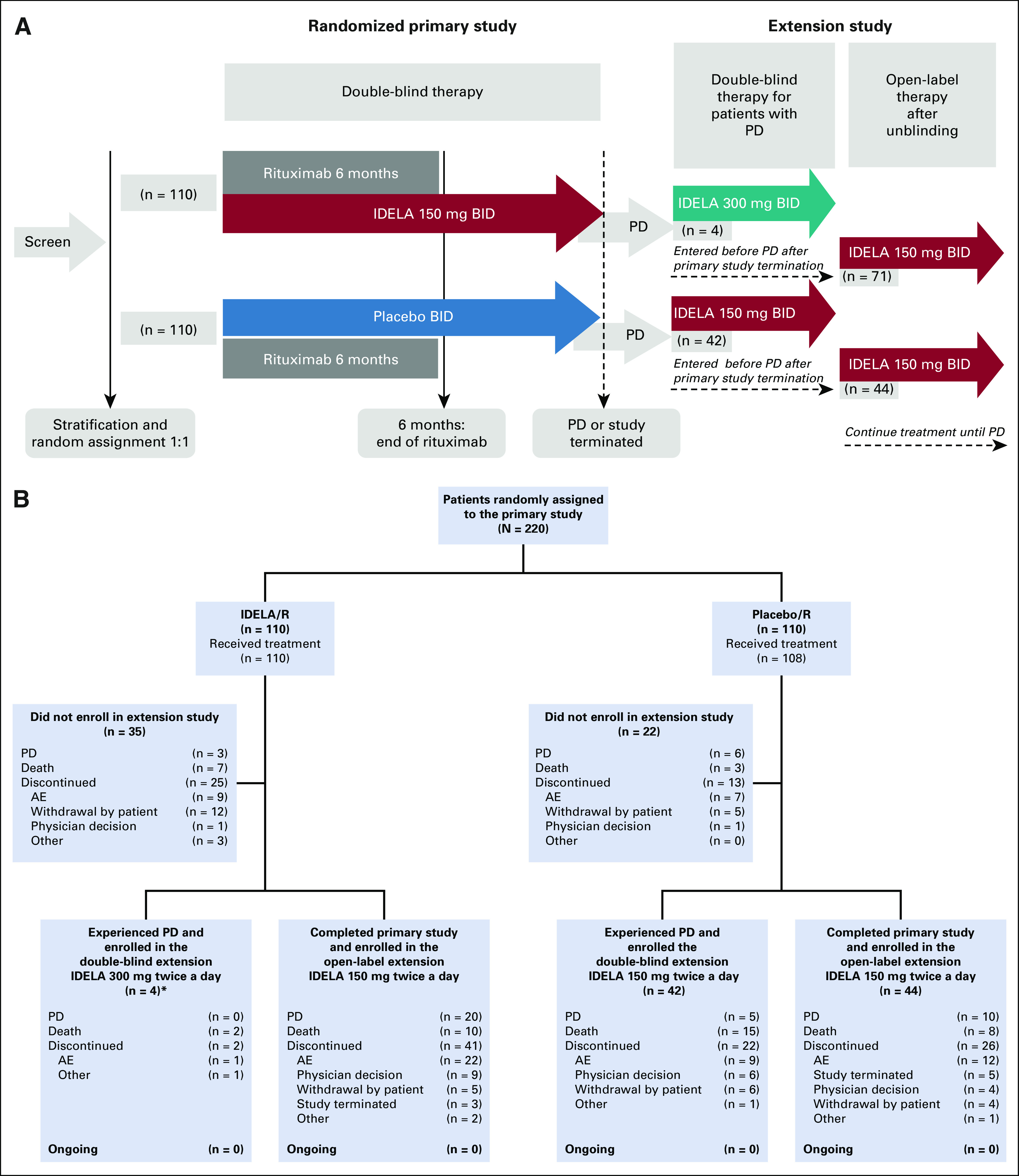
(A) Study flow. (B) Patient flow. (*)For all analyses in this report, the four patients who experienced PD during treatment with IDELA/R and received single-agent IDELA 300 mg twice a day in the double-blind part of the extension study were pooled together with patients treated with IDELA/R who did not experience PD, and, at study termination, transitioned to open-label IDELA 150 mg twice a day in the extension study. AE, adverse event; IDELA, idelalisib; PD, progressive disease; R, rituximab.
