Figure 2.
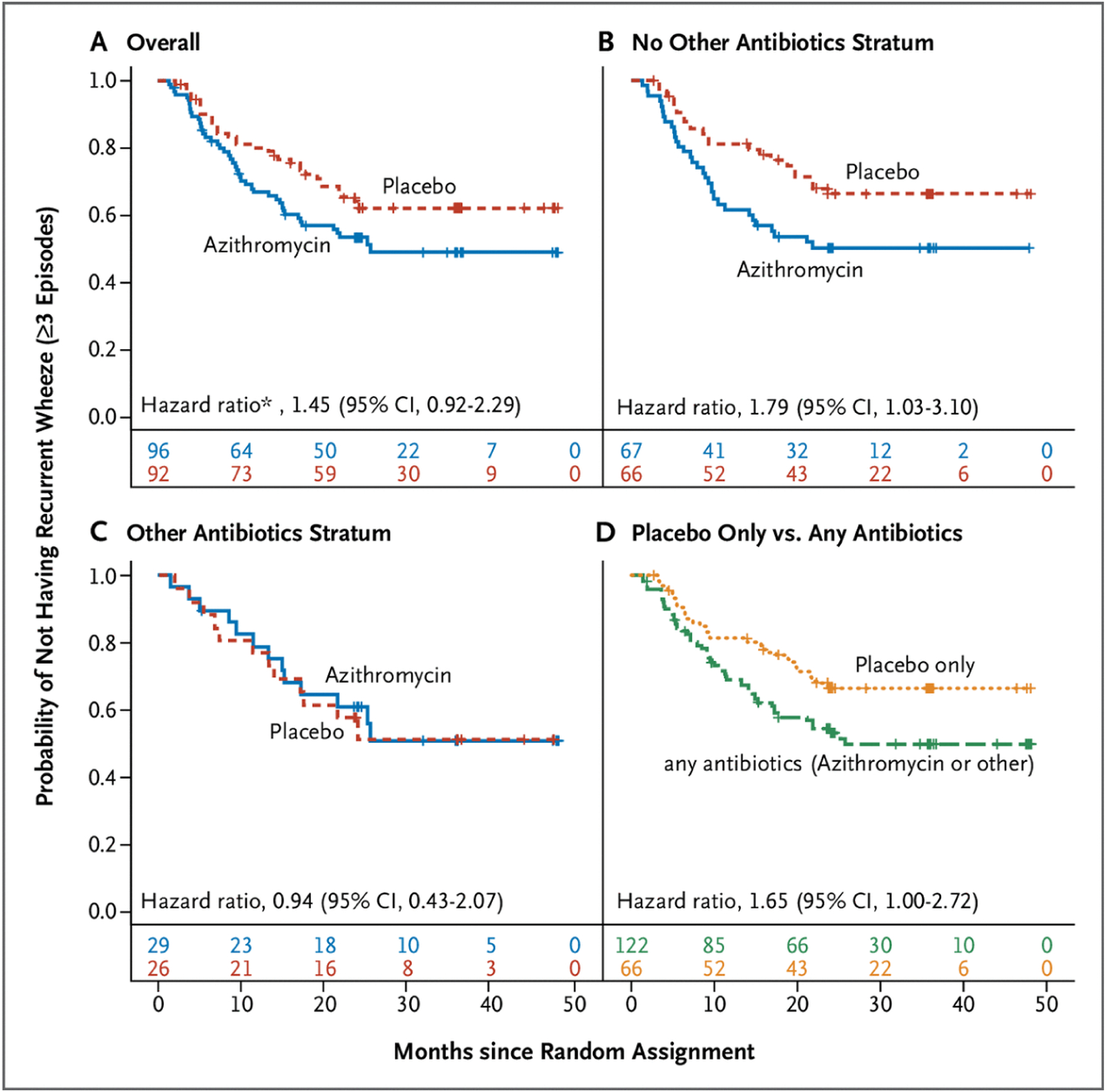
Time to Post–Respiratory Syncytial Virus Third Episode of Wheezing (Recurrent Wheeze).
A, Among the whole cohort (the primary outcome). B, Among participants who had not received open-label nonmacrolide antibiotics (“other antibiotics”) at or within 14 days before random assignment. C, Among participants who had received open-label nonmacrolide antibiotics (“other antibiotics”) at or within 14 days before random assignment. D, A post hoc analysis of the time to recurrent wheeze among participants who received placebo and had not received other antibiotics (i.e., had not received any antibiotic) versus participants who received any antibiotics (azithromycin and/or open-label nonmacrolide antibiotic). CI denotes confidence interval. Numbers below the x-axis in each graph indicate the number at risk at each time point.
* Hazard ratio adjusted for parental history of asthma, race, tobacco smoke exposure, and pet exposure.
