Fig 1. E3 ligase activity is conserved among XopL homologs from different xanthomonads.
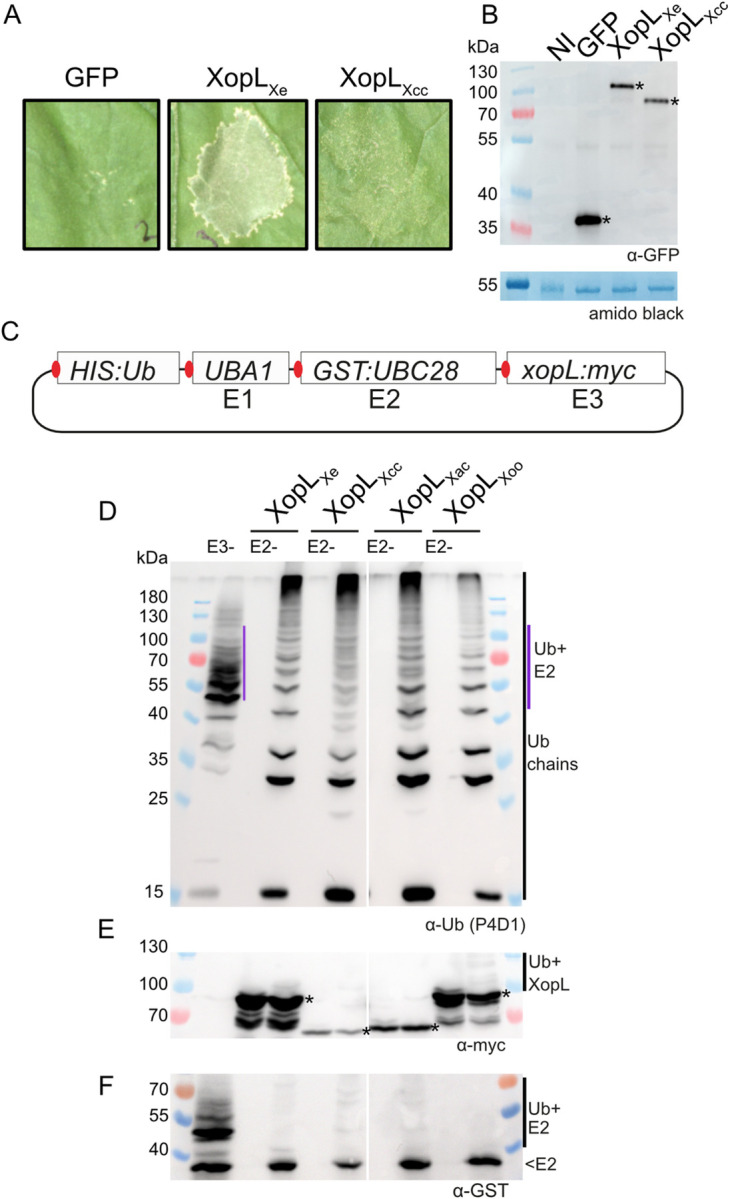
(A) Agroinfection of N. benthamiana leaves to express GFP-myc and XopL proteins from Xanthomonas strains Xe 85–10 (XopLXe) and Xcc 8004 (XopLXcc) fused to a C-terminal GFP (OD of 0.8). Plant reactions were monitored 5 dpi. (B) Western blot analysis of protein extracts isolated 2 dpi from samples depicted in (A). One ‘not inoculated’ (NI) sample was included as a negative control. Signals were detected using a GFP-specific antibody (*). The left side of the blot shows protein mass in kDa. Amido black staining is shown as a loading control. (C) Schematic of the UbiGate plasmid used to test for E3 ligase activity of XopL proteins. Components of the A. thaliana ubiquitination machinery (affinity-tagged for detection by western blot), UBIQUITIN 10 (HIS:Ub), UBIQUITIN ACTIVATING ENZYME 1 (UBA1) and UBIQUITIN CONJUGATING ENZYME 28 (GST:UBC28) were expressed from a single, IPTG-inducible plasmid together with xopL coding sequences. Each translational unit was equipped with an independent ribosome binding site (red). (D-F) Western blot analysis of protein extracts isolated from E. coli expressing different XopL proteins. Controls were samples lacking the E3 ligase (E3-) or the E2 enzyme (E2-). The XopL protein tested is indicated above the designated lanes. (D) Ubiquitin was detected using the P4D1 antibody. Polyubiquitin chains are indicated by a black line. E2-ubiquitin (Ub+E2) conjugates are visible in E3- control sample (purple line). (E) C-terminally tagged XopL proteins were detected with a myc-specific antibody (expected size indicated with ‘*’). In some cases, autoubiquitination is detectable (Ub+XopL). (F) N-terminally-tagged E2 enzyme was detected using a GST-specific antibody. Samples were run on different gels to clearly visualize ubiquitin and other proteins. Protein mass is expressed in kDa.
