Figure 1. Non-Invasive Diagnostics and Respective Evidentiary Support to Identify Rejection Phenotypes.
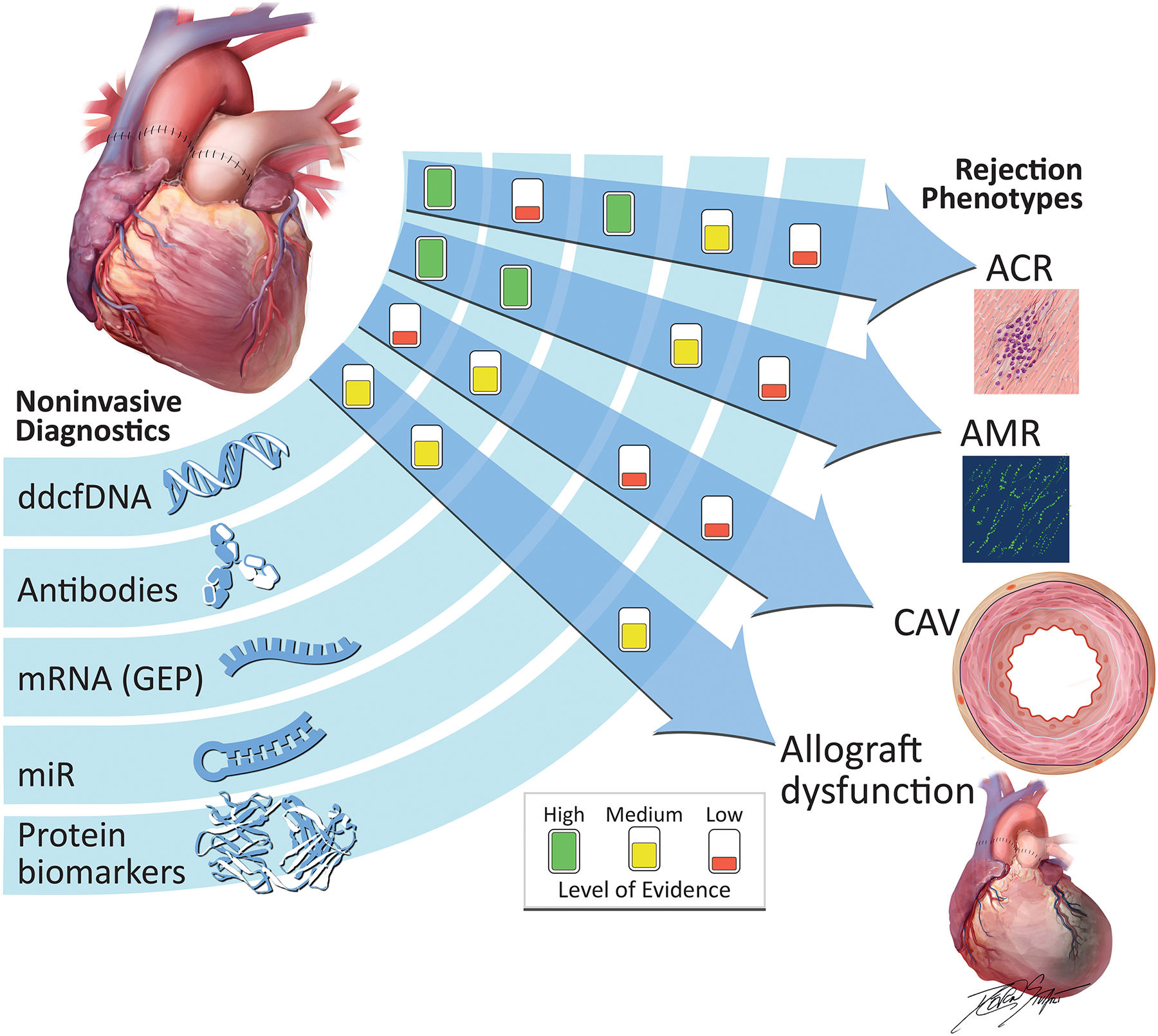
Differing levels of evidence exist for non-invasive diagnostic modalities to detect post-transplant rejection, per recommendations of a recent consensus guideline. Associations are denoted as clinical discovery, clinical validity and clinical utility. Only the association between GEP and ACR meets clinical utility criteria, given the IMAGE randomized, controlled trial. The GRAfT, multicenter prospective study resulted in clinical validity for dd-cfDNA’s ability to detect ACR and AMR. The presence of HLA donor-specific antibodies have been associated with AMR, CAV, and allograft dysfunction. Recent microRNA work has provided evidence of its role in diagnosis of ACR and AMR. Finally, proteomic evaluation has identified circulating biomarkers of allograft dysfunction and CAV.
ACR, acute cellular rejection; AMR, antibody-mediated rejection; CAV, coronary allograft vasculopathy; dd-cfDNA, donor-derived cell-free DNA; HLA, human leukocyte antigen; mRNA, messenger RNA; miR, microRNA.
