Figure 2. Featured Donor-Derived Cell-Free DNA Publications and their Contributions to the Evaluation of Cardiac Allograft Rejection.
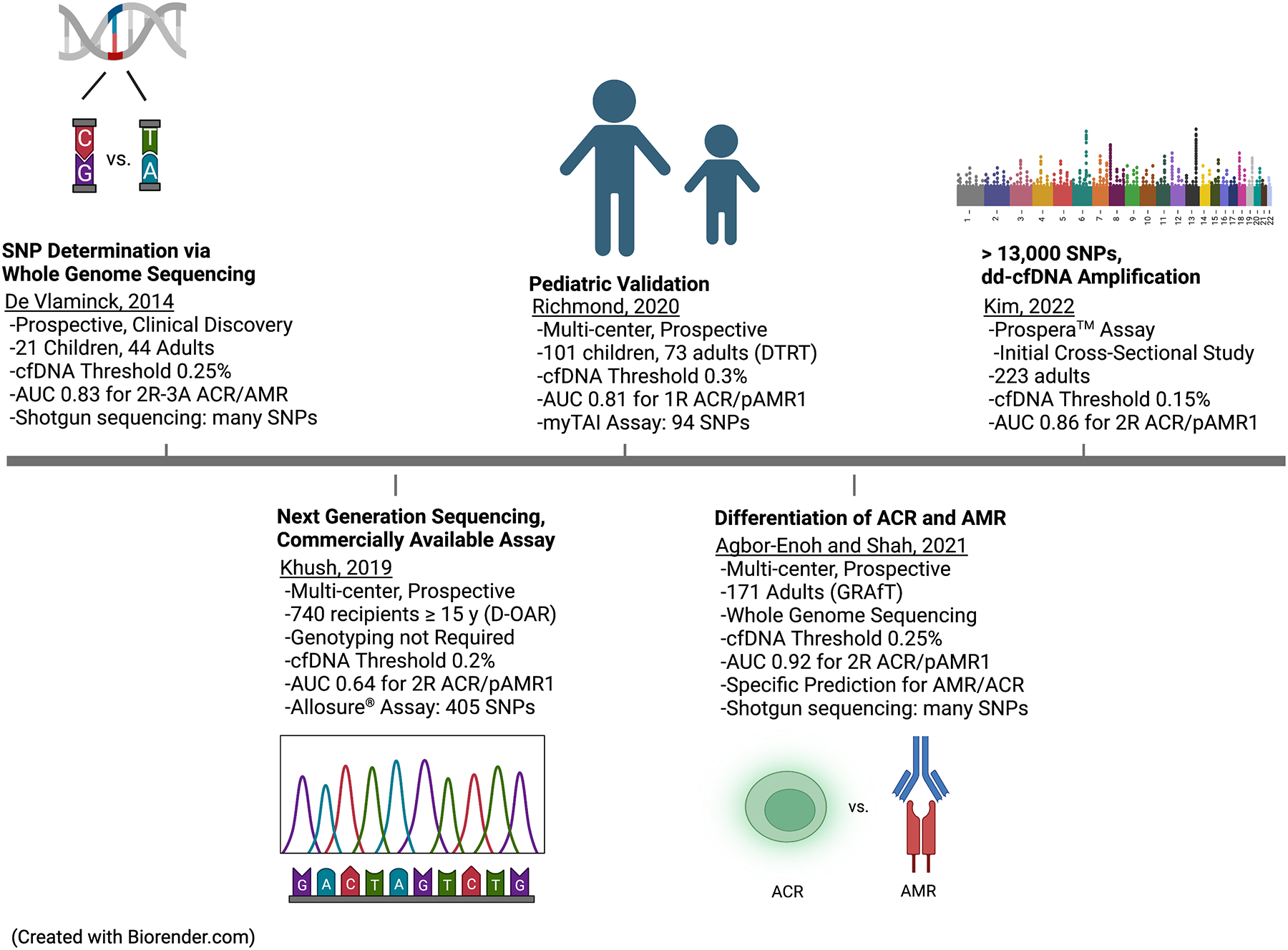
This represents the time course of major studies which contributed to our understanding of the potential clinical applications of dd-cfDNA in the diagnosis of cardiac allograft rejection. The contributions include the initial research assay or clinical discovery work performed at Stanford University in 2014. Clinical validation studies by the GRAfT consortium showed high diagnostic performance of dd-cfDNA when considering ACR and AMR separately. Expansion of research into a large cohort of pediatric patients by the DTRT group. And finally, use of commercial assays that do not require donor or recipient genotyping.
ACR, acute cellular rejection; AMR, antibody-mediated rejection; AUC, area under the curve; cfDNA, cell-free DNA; DTRT, DNA-Based Transplant Rejection
Test; GRAfT, Genomic Research Alliance for Transplantation; SNP, single nucleotide polymorphisms.
