Figure 2. Optimization of carbon–carbon bond formation.
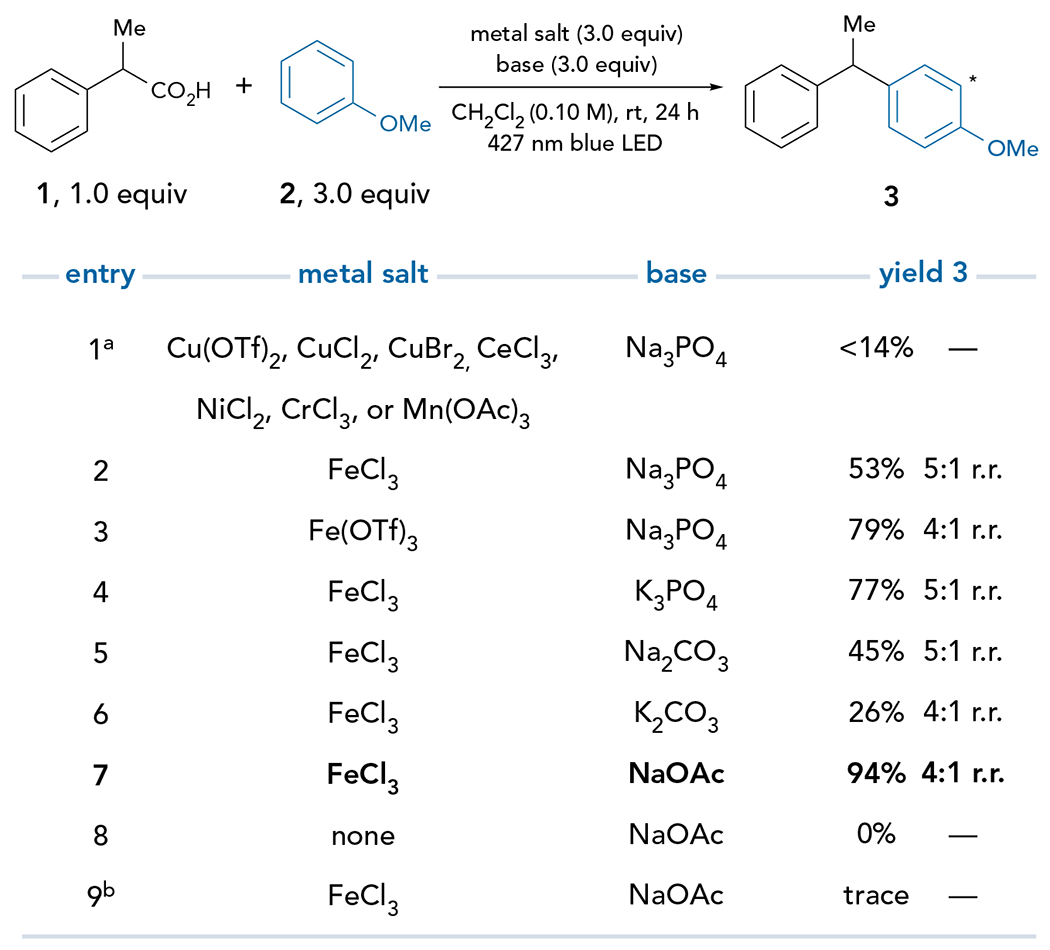
Reaction conducted using metal salt (3.0 equiv), base (3.0 equiv), nucleophile 2 (3.0 equiv), and carboxylic acid 1 (0.1 mmol) in CH2Cl2 (0.10 M) setup under inert atmosphere and irradiated with a 427 nm blue Kessil Lamp at RT for 24 h. Yields were determined by 1H NMR analysis of the crude reaction mixture using 1-methylnaphthalene as an internal standard. aSee supplemental information for full reaction details. bReaction vessel was wrapped in aluminum foil.
