Figure 6. The intermediary of an alkyl chloride enables further diversification of carboxylic acid feedstocks.
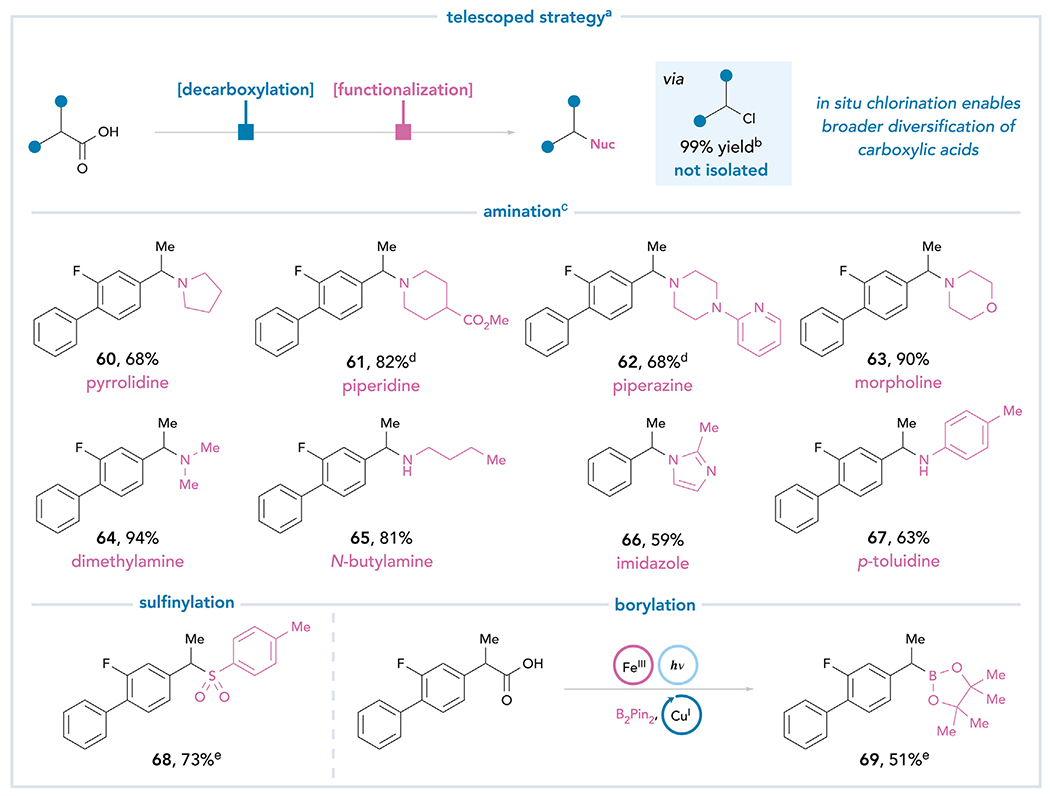
All yields are isolated unless otherwise noted. aReaction conditions: FeCl3 (3.0 equiv), Na3PO4 (3.0 equiv), carboxylic acid (1.0 equiv), and MeCN (0.10 M), 24 h, rt, 427 nm blue LED. bYield was determined by 1H NMR analysis of the crude reaction mixture using 1-methylnaphthalene as an internal standard. cAfter irradiation, amine (10 equiv) and KI (1.5 equiv) were added directly to the reaction mixture and heated to 70°C overnight. d7.0 equiv of the amine. eSee supplemental information for experimental details.
