Abstract
Nitrogen-containing heterocycles are the key components in many pharmaceuticals and functional materials. In this study, we report a transition metal-catalyzed high-order reaction sequence for synthesizing a structurally unique N-center 5,6,7-perifused cycle (NCPC). The key characteristics include the formation of a seven-membered ring by the 8π electrocyclization of various alkenes and aromatic heterocycles as π-components, in which metal carbene species are generated that further induce the cleavage of the α-C-H or -C-C bond. Specifically, the latter can react with various nucleophilic reagents containing -O, -S, -N, and -C. The stereo-controlled late-stage modification of some complicated pharmaceuticals indicates the versatility of this protocol.
Subject terms: Drug discovery and development, Catalyst synthesis, Synthetic chemistry methodology
The lack of efficient and diverse synthesis strategy has hindered the study of perifused cycles. Here, the authors report a metal-catalyzed cascade electrocyclization to access 5,6,7-perifused cycles, and demonstrated the versatility of this protocol in the late-stage modification of pharmaceuticals.
Introduction
Natural products are often used as the lead compounds1–5 in designing or searching for pharmaceuticals. For example, 5,6,7-perifused carboncycle compounds, tricyclic compounds that share the same center atom, form the core skeleton in many bioactive natural products (Fig. 1a)6–9. These scaffolds might be used as the lead structures for guiding the synthesis of analogs, thus, promoting drug development. In contrast, N-center perifused cycles (NCPC) might further reveal interesting perspectives for fundamental studies and technological applications because the nitrogen atom has higher electronegativity and smaller van der Waals radius than the carbon atom, which can increase molecular polarity and water solubility10,11, thus improving molecular selectivity12 and availability (Fig. 1b). However, when the nitrogen atom is located in the central position of the tricyclic system, the valence state and non-bonding ion pairs can increase the difficulty of synthesis. Specifically, when the medium ring is involved, the difficulty in synthesis might be further increased due to its high torsional strain, unfavorable entropic and enthalpic factors13–18, and other reasons. Previous routes for synthesizing NCPC start from α-formyl-pyrrole19–22 or -pyridine23 as raw materials involved a cumbersome multi-step strategy, and the related methods were also rare (Fig. 1c). In some contexts, the lack of a simple catalytic protocol to access skeletons might also hinder their applications in synthetic chemistry. Therefore, an efficient synthetic method needs to be developed to further extract or elucidate their application in organic synthesis.
Fig. 1. Background and our proposal.
a Representative tricarbocycles natural products. b Carbon atom vs nitrogen atom. c Traditional methodology of synthesis the NCPC. d Our strategy to build NCPC.
Transition metal-catalyzed cascade cyclization24–26 represents an efficient strategy for assembling various polycyclic systems. As shown in Fig. 1d, we speculate that the metal-catalyzed 5-endo-cyclization of pyridine-alkyne might form zwitterionic vinyl metal species27–40 that might resonate to become a reactive conjugated 8π electronic system and a metal carbene41–43. Electrocyclization reactions44–49 can be driven by heating and are not affected by external conditions. However, the reactivity of metal carbene is closely related to the reaction system. Thus, electrocyclization might be conducted for constructing seven-membered ring structures, promoting the formation of NCPC metal carbene intermediates that provide a great opportunity for applying reactive metal carbene species as a common intermediate for the divergent synthesis of substituted NCPC with high efficiency and flexibility. The metal carbene is a highly versatile intermediate, and NCPC metal carbene provide synthetic chemists access to various structural motifs. The present method might be a suitable alternative for synthesizing NCPC derivatives and could be a way to produce the related N-center substituted series of natural products, which cannot be prepared using other means.
In this work, we report the results of metal-catalyzed cascade cyclization for the divergent synthesis of NCPC. The protocol includes the following points. (1) The final seven-membered ring is constructed by 8π electrocyclization, which is interesting due to its value in addressing the differences in the formation of a medium ring in complicated polycyclic systems. (2) The process includes multi-chemical events, which help in constructing NCPC structural units directly and rapidly through a high-order reaction sequence. (3) The “non-classical” tricyclic metal carbene species are used as a common intermediate while synthesizing the NCPC. (4) The structurally unique NCPC might be used as a platform model for discovering some synthetically useful compounds. (5) This study might provide useful information for designing perifused ring analogs with potent activity.
Results
Optimization of reaction conditions
We used the propargylic derivative of 6-vinylpyridine 1a as our model substrate for cleaving α-C–H triggered by metal carbene (Table 1). Initially, we screened various metals based on the known alkynophilicity of gold50,51, silver52,53, and copper compounds54–56. When the solution of 1a (0.05 mmol) in toluene was reacted using chloro(triphenylphosphine) gold (AuPPh3Cl) as the catalyst at 90 °С, the expected NCPC product 2a was formed with a 31% yield (Table 1, entry 1), and its structure was confirmed by X-ray single crystal diffraction analysis. Because gold and silver salts combine to release highly active gold species, improving the catalytic ability of the gold catalyst and then combining AuPPh3Cl and AgOTf showed high reactivity and significantly improved the yield to 60% (Table 1, entry 2). Switching Au(I) to various silver salts, such as AgSbF6, AgOTf, and Ag3PO4 yielded only trace amounts of the product (Table 1, entries 3−5). In contrast, various copper salts yielded the expected products in high yields in all cases (Table 1, entries 6−9). Among them, CuTc was used to produce the cyclization product with the highest yield (96%) (Table 1, entry 9). Subsequently, decreasing the reaction temperature from 90 °С to 60 °С decreased the yield (Table 1, entry 9). Finally, applying commonly used solvents, such as 1,2-DCE, THF and DMF, did not have a better effect (Table 1, entries 10−12).
Table 1.
Optimization of the reaction conditionsa,b
| Entry | Catalyst | Solvents | T (°С) | Yield (%) |
|---|---|---|---|---|
| 1 | AuPPh3Cl | PhMe | 90 | 31 |
| 2 | AuPPh3Cl + AgSbF6 | PhMe | 90 | 60 |
| 3 | AgSbF6 | PhMe | 90 | Trace |
| 4 | AgOTf | PhMe | 90 | Trace |
| 5 | Ag3PO4 | PhMe | 90 | Trace |
| 6 | CuSO4 | PhMe | 90 | 75 |
| 7 | Cu(OAc)2 | PhMe | 90 | 84 |
| 8 | Cu(CH3CN)4PF6 | PhMe | 90 | 68 |
| 9 | CuTc | PhMe | 90 (60) | 96 (23)c |
| 10 | CuTc | 1,2-DCE | 90 | 65 |
| 11 | CuTc | THF | 90 | 45 |
| 12 | CuTc | DMF | 90 | 31 |
aAll reactions of 1a (0.05 mmol, 1.0 equiv.) and catalyst (0.005 mmol, 10 mol%) were performed in solvent (1.0 mL) at 90 °С for 0.5 h.
bIsolated yield.
cReaction was performed at 60 °С.
Reaction scope of Cu-catalyzed α-C–H bond cleavage
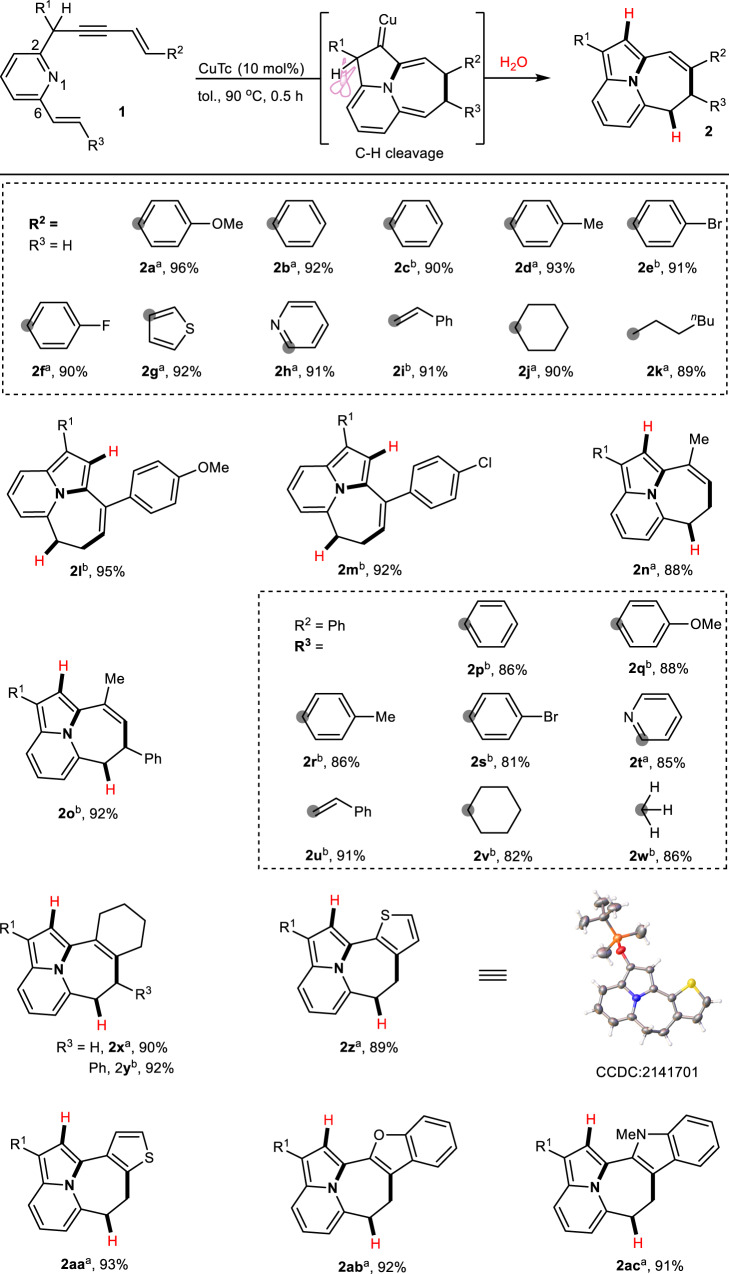
After establishing the optimized reaction conditions, we investigated the scope of NCPCs under Cu-catalysis (Fig. 2). The eneyne moieties possessing different aryl (2a−2 f), heteroaryl (2 g and 2 h), alkenyl (2i), and alkyl (2j and 2k) substituents at the end of the alkene underwent smooth conversion to yield the corresponding NCPCs with excellent yields. Adding a substituent at the internal position of the eneyne moieties (2 l−2n) also produced the desired products in high yields. The generality of this transformation was further extended by placing various substituents, including aryl (2o−2 s), heteroaryl (2t), alkenyl (2 u), and alkyl (2 v and 2w) substituents, on the alkenyl segment at the C6 position of pyridine, where these substrates could be converted into the expected products in high yields. Halogen-containing aryl-substituents (2e, 2 m and 2 s) were also competent in the cascade cyclization process, providing an additional functional handle for further modification.
Fig. 2. Substrate scope of α-C–H bond cleavage.
Reaction Conditions: 1 (0.05 mmol) and CuTc (0.005 mmol) in toluene (1.0 mL) at 90 °С for 0.5 h. aR1 = OTBS, bR1 = OAc. Isolated yields are provided.
We further investigated the generality of this transformation by using the annular eneynes that were efficiently converted into the corresponding tetracyclic products (2x and 2 y) with excellent yields. When the annular eneyne was replaced by thiophene, the transformation was successful irrespective of whether the α-position or β-position of thiophene was used. Through this process, tetracycle products were formed with a fused aromatic ring (2z and 2aa). The O- or N-containing aromatic compounds, such as benzofuran and indole, also underwent the cascade cyclization sequence, thus providing access to fused pentacyclic scaffolds (2ab and 2ac). To confirm the structure of the products, a single crystal X-ray structure of product 2z was obtained, and the structure of other products was deduced from their NMR spectra. In addition, we carried out this cascade process by using benzene ring as π-component. To our disappointment, the reaction of the phenyl group as the π-component was interrupted during the 5-endo-cyclization step, and subsequent 8π electrocyclization of benzene did not proceed as predicted (please see Supplementary Information, Page 28). It may be attributed to the weak aromatic system of the electron-rich furan or thiophene due to uneven charge distribution. The inherent strong aromatic property of benzene makes it difficult to dearomatize in 8π electrocyclization process, resulting that the cascade sequence was interrupted in the 5-endo-cyclization step.
A part of this study involved research on the α-C–C bond cleavage triggered by metal carbene, in which the highly versatile carbocation intermediate was formed and subsequently reacted with various nucleophilic reagents, leading to the formation of various nucleophilic substituted NCPC products. The three-membered cycle showed high structural strain57,58, which provided a dynamic momentum and a thermodynamic driving force for α-C–C cleavage. Using this, the substrate bearing a cyclopropyl between pyridine and alkyne (3a) was synthesized, and the corresponding reaction was conducted to determine whether the ring-opening nucleophilic substitution reaction would occur through the α-C–C cleavage at the α-position of metal carbene. To our delight, we found that the cascade sequence also formed the expecting product (4a) with an 11% yield using CuTc as the catalyst and NaOAc as the nucleophilic reagent. In principle, the success of this divergent synthesis process depended on subtle differences in the reactivity between the starting material and the metal catalyst because both metal carbene of different reactivity triggered different transformations. We focused mainly on screening the metal catalyst to further improve the yield. After extensive screening, we found that the silver salts highly stimulated the α-C–C cleavage. Finally, using silver phosphate as the catalyst and TEBA as an additive in the presence of NaOAc, product 4a was formed with an 80% yield (Detailed conditions screening, please see Supplementary Information, Supplementary Table 1). In general, the metal carbene triggered the nucleophilic ring-opening process of the three-membered ring, which resulted in the complete release of ring strain. This process is thermodynamically favorable compared to the formation of a four-membered ring through 1,2-alkyl migration.
Reaction scope of Ag-catalyzed α-C–C bond cleavage
Next, we investigated the scope of the cascade sequence (Fig. 3). When the phenyl at the end of the eneyne was replaced with thienyl, the ring-opening reaction proceeded slowly to generate the thienyl-substituted product (4b) with an 89% yield. Besides aromatic rings, the alkenyl (4c) or alkyl (4d and 4e) substitutions at the same position were also suitable substrates, affording the corresponding products in high yields. Similarly, the C2 and C3 positions of thiophene could be changed to obtain the desired tetracyclic products (4 f and 4 g) with yields of 69% and 78%, respectively. The conversion of thiophene into indole was tolerated and led to the formation of the pentacyclic product (4 h) with a 71% yield. When the cyclopropyl with a 4-Me-phenyl (4i) or 4-F-phenyl (4j), was exposed to the standard reaction condition to determine the effect of the substituents, the results indicated that the latter led to a lower yield probably because the para-F-substitution was not favorable for the stability of the carbocation intermediate. Additionally, using sodium formate as a nucleophilic reagent also generated the desired product (4k) with a satisfactory yield. Although the nucleophilicity of phenoxyl was relatively weak, it led to the corresponding oxo-substitution of NCPC (4 l) with a 52% yield.
Fig. 3. Substrate scope of α-C–C bond cleavage.
Reaction Conditions: 2 (0.1 mmol), Ag3PO4 (0.02 mmol), TEBA (0.2 mmol) and nucleophile (0.3 mmol) in CHCl3 (2.0 mL) at 100 °С for 24 h. Isolated yields are provided. TEBA: Benzyltriethylammonium chloride.
Subsequently, the reaction was conducted to test whether the transformation could be performed using different types of nucleophilic reagents. The results indicated that 2-thiol-benzothiazole as a nucleophilic reagent could produce the corresponding product (4 m) with a 63% yield. The nitrogen-containing nucleophiles, such as aniline, sodium azide, 1,2,3-triazole, and tetrazole, were also investigated, and the results indicated that all nucleophilic reagents reacted effectively under standard conditions, resulting in the formation of the desired products (4n−4q) with satisfactory yields. Regarding the nucleophilicity of the carbon atom, we used the sodium salt of malononitrile and indole as nucleophilic reagents. Both substrates participated in this intermolecular ring-opening substitution reaction, forming the desired products (4r and 4 s) with satisfactory yields.
The late-stage modification of pharmaceuticals59,60 is of significant importance for the discovering drugs or improving bioactivity (Fig. 4). To further demonstrate the potential of this strategy, we examined the application of the ring-opening nucleophilic substitution in the late-stage modification of biologically active small molecules. The different marketed pharmaceuticals, including structurally complicated Gemfibrozil, Idometacin, Estrone, Riluzole, and Candesartan cilexetil, accessing divergent products (4t−4x) with satisfactory yields. These modifications can also provide key advantages from the perspective of medicinal chemistry. The catalytic system showed high compatibility with several nucleophilic reagents and rapidly established molecular complexity and diversity.
Fig. 4. Late-stage modification of pharmaceuticals.
Introducing the 5,6,7-perifused tricycle into pharmaceuticals through oxo or aza-nucleophilic ring-opening of α-metal carbene cyclopropane.
Synthetic utility and mechanistic studies
To demonstrate the practicability of our methodology, the gram-scale reactions of 1c and 3a were conducted, and the products 2c and 4a were formed with yield of 81% and 71% (Fig. 5a), respectively. Additionally, the hydrolysis of product 4a occurred in the presence of potassium carbonate to deliver 4a-1 with a 96% yield. Next, oxidative dehydrogenation proceeded sluggishly; DDQ (1,2-dihloro-4,5-dicyanobenzoquinone) was used as an oxidant, which allowed the conversion of 4a into 4a-2 rather than cycloazine (Fig. 5b). This selective oxidation can be attributed to the difference in C–H bond energy. The α-position of the oxygen atom typically has a lower C–H bond energy compared to a normal C–H bond61,62, making it more susceptible to radical generation during the oxidation by DDQ. Additionally, the tertiary radical on the side chain is more stable than the secondary radical on the tricyclic skeleton.
Fig. 5. Synthetic utility.
a Gram-scale synthesis. b Synthetic transformations.
To understand this mechanism and elucidate some specific processes of the reaction, several control experiments were conducted. The deuterated starting material 1a’ was exposed to the standard conditions. The NMR spectra showed that the reaction generated product 2a rather than the deuterated product 2a’ (Fig. 6a), indicating that the intramolecular 1, 2-hydride shift triggered by metal carbene was not involved in the process. To identify the H source, the nondeuterated substrate 1a was reacted with a mixture of toluene with D2O (toluene/D2O = 1/0.2), and the reaction produced the deuterated product 2a”; the C4 and C2’ positions were deuterated at a 90% and 100% deuterated ratio, respectively (Fig. 6b). These results suggested that the reaction underwent protonation twice in the presence of the proton donor that came from H2O in the solvent.
Fig. 6. Control experiments and possible mechanism.
a Control experiment of substrate 1a’. b Control experiment of D2O as mixed solvent. c Possible mechanism for the formation of NCPC.
Based on the above mechanistic investigations and the results of our experiments, we proposed a plausible catalytic cycle (Fig. 6c). The 5-endo-cyclizations of substrates 1 and 3 proceeded under metal catalysis to form the intermediates I and I′, which formed the common metal carbene intermediates (II) and (II′), respectively, via 8π electrocyclization. The results of our control experiment showed that intermediate II triggered deprotonation through the cleavage of C–H to produce vinyl metal intermediate III, which allowed protonation in the presence of H2O to produce intermediate IV along with the release of the metal catalyst. Intermediate II′ underwent ring-opening via a C–C bond cleavage, which involved nucleophilic substitution along with the generation of vinyl metal intermediate III′. A similar protonation process formed intermediate IV′. These two polyene intermediates IV and IV′ underwent further isomerization to form final products 2 and 4, respectively. The whole reaction underwent intermolecular protonation twice rather than intramolecular H-migration.
In conclusion, we showed the unique synthetic potential of cascade cyclization for constructing structurally diverse N-center polycycle skeletons. The key feature of this transformation was that the 8π electrocyclization could be used to construct the medium ring along with the generation of NCPC metal carbene, which served as versatile building blocks for α-C–H or -C–C cleavage. This procedure is an effective way to rapidly access structurally divergent nitrogen-containing polycyclic products. The 8π electrocyclization might also be used to develop efficient strategies for rapidly constructing a library of fused polycycles. These structurally attractive NCPC skeletons might be interesting since they can help further enhance the utility of this powerful synthetic strategy.
Methods
General procedure for α-C–H bond cleavage
The freshly prepared 1 (0.05 mmol, 1.0 equiv), CuTc (10.0 mol%), and toluene (1.0 mL) were added to a 5.0 mL round bottom flask at room temperature, the reaction mixture was stirred at 90 °C for 0.5 h and then cooled to room temperature. After completion, the reaction mixture was filtered, and the organic solution was concentrated under vacuo, residue was purified by column chromatography on alkaline alumina to afford the desired product.
General procedure for α-C–C bond cleavage
Ag3PO4 (20.0 mol%), TEBA (0.2 mmol, 2.0 equiv) and nucleophilic reagent (0.3 mmol, 3.0 equiv) were added to a solution of freshly prepared 3 (0.1 mmol, 1.0 equiv) in CHCl3 (2.0 mL) for a Schlenk tube at room temperature, the reaction mixture was stirred at 100 °C for 24 h and then cooled to room temperature. After completion, the reaction mixture was filtered, and the organic solution was concentrated under vacuo, residue was purified by column chromatography on silica gel to afford the desired product.
Supplementary information
Acknowledgements
The authors thank NSFC (Grant 21772019 to B.L.), acknowledge the support from the Venture & Innovation Support Program for Chongqing Overseas Returnees (cx2019007 and cx2020047 to B.L.), and the Basic and Frontier Research Project of Chongqing (CSTB2022NSCQ-MSX0320 to B.L.) and the Analytical and Testing Centers of Chongqing University are gratefully acknowledged for instrumental facilities.
Author contributions
J.H. performed most of the experiments, including the synthesis of substrates, collection of the data, mechanistic studies and analyzed the data. Y.Y. and Y.G. helped synthesize some of the substrates. X.T. and Y.T. repeated the results. B.L. conceived the study and wrote the paper. All authors discussed the results and approved the final version of the manuscript.
Peer review
Peer review information
Nature Communications thanks Liejin Zhou and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Data availability
The authors declare that all data generated in this study are available within the paper and its Supplementary Information files. The X-ray crystallographic coordinates for structures reported in this article have been deposited at the Cambridge Crystallographic Data Center (2a: CCDC 2141700; 2z: CCDC 2141701; 3a: CCDC 2239536; 4 m: CCDC 2141703). These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif. Any additional detail can be requested from the corresponding authors.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-023-40801-0.
References
- 1.Newman DJ, Giddings L-A. Natural products as leads to antitumor drugs. Phytochem. Rev. 2014;13:123–137. [Google Scholar]
- 2.Shen B. A new golden age of natural products drug discovery. Cell. 2015;163:1297–1300. doi: 10.1016/j.cell.2015.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rodrigues T, Reker D, Schneider P, Schneider G. Counting on natural products for drug design. Nat. Chem. 2016;8:531–541. doi: 10.1038/nchem.2479. [DOI] [PubMed] [Google Scholar]
- 4.Khalaf RA. Exploring natural products as a source for antidiabetic lead compounds and possible lead optimization. Curr. Topics Med. Chem. 2016;16:2549–2561. doi: 10.2174/1568026616666160414123602. [DOI] [PubMed] [Google Scholar]
- 5.Xu Y, et al. Antitussive and anti-inflammatory dual-active agents developed from natural product lead compound 1-methylhydantoin. Molecules. 2019;24:2355. doi: 10.3390/molecules24132355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baker R, Walmsley S. Soldier defense secretions of the south American termites Cortaritermes Silvestri, Nasutitermes SP N.D and Nasutitermes Kemneri. Tetrahedron. 1982;38:1899–1910. [Google Scholar]
- 7.Bohlmann F, Ahmed M, Jakupovic J, King RM, Robinson H. Dimeric sesquiterpene lactones and kolavane derivatives from Gochnatia paniculata. Phytochemistry. 1983;22:191–195. [Google Scholar]
- 8.Wang Y, et al. Ainsliatrimers A and B, the first two guaianolide trimers from Ainsliaea fulvioides. Org. Lett. 2008;10:5517–5520. doi: 10.1021/ol802249z. [DOI] [PubMed] [Google Scholar]
- 9.Ni G, et al. Mannolides A–C with an Intact diterpenoid skeleton providing insights on the biosynthesis of antitumor cephalotaxus troponoids. Org. Lett. 2016;18:1880–1883. doi: 10.1021/acs.orglett.6b00653. [DOI] [PubMed] [Google Scholar]
- 10.Ji Y-Y, et al. Introducing nitrogen atoms to amidoalkylindoles: potent and selective cannabinoid type 2 receptor agonists with improved aqueous solubility. Med. Chem. Commun. 2019;10:2131–2139. doi: 10.1039/c9md00411d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cosgrove B, Down K, Bertrand S, Tomkinson NCO, Barker MD. Investigating the effects of the core nitrogen atom configuration on the thermodynamic solubility of 6,5-bicyclic heterocycles. Bioorg. Med. Chem. Lett. 2021;33:127752. doi: 10.1016/j.bmcl.2020.127752. [DOI] [PubMed] [Google Scholar]
- 12.Vögerl K, et al. Synthesis and biological investigation of phenothiazine-based benzhydroxamic acids as selective histone deacetylase 6 inhibitors. J. Med. Chem. 2019;62:1138–1166. doi: 10.1021/acs.jmedchem.8b01090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Molander GA. Diverse methods for medium ring synthesis. Acc. Chem. Res. 1998;31:603–609. [Google Scholar]
- 14.Yet L. Metal-mediated synthesis of medium-sized rings. Chem. Rev. 2000;100:2963–3008. doi: 10.1021/cr990407q. [DOI] [PubMed] [Google Scholar]
- 15.Itoh Y, et al. Efficient formation of ring structures utilizing multisite activation by indium catalysis. J. Am. Chem. Soc. 2008;130:17161–17167. doi: 10.1021/ja805657h. [DOI] [PubMed] [Google Scholar]
- 16.Sharma A, Appukkuttan P, Van der Eycken E. Microwave-assisted synthesis of medium-sized heterocycles. Chem. Commun. 2012;48:1623–1637. doi: 10.1039/c1cc15238f. [DOI] [PubMed] [Google Scholar]
- 17.Li L, et al. Radical aryl migration enables diversity-oriented synthesis of structurally diverse medium/macro- or bridged-rings. Nat. Commun. 2016;7:13852. doi: 10.1038/ncomms13852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Uno H, Kawai K, Shiro M, Shibata N. Modular synthesis of medium-sized fluorinated and nonfluorinated heterocyclic lactones by sequential CN-bond-cleaving ring expansion under Pd catalysis. ACS Catal. 2020;10:14117–14126. [Google Scholar]
- 19.Flitsch W, Müter B. Cycl[4.3.2]azin-4,5-dicarbonsäure-diäthylester. Angew. Chem. 1973;85:543. [Google Scholar]
- 20.Flitsch W, Gurke A, Müter B. Zur Chemie der Cycl[4.3.2]azine. Chem. Ber. 1975;108:2969–2977. [Google Scholar]
- 21.Flitsch W, Krämer U. Cyclazines and related N-bridged annulenes. Adv. Heterocycl. Chem. 1978;22:321–365. [Google Scholar]
- 22.Flitsch W, Mukidjam E. Beiträge zur Chemie der Cycl[4.3.2]azine. Chem. Ber. 1979;112:3577–3588. [Google Scholar]
- 23.Miki Y, Hachiken H, Yoshikawa M, Takemura S, Ikeda M. A new entry to [2.3.4]cyclazines. Heterocycles. 1991;32:655–658. [Google Scholar]
- 24.Malacria M. Selective preparation of complex polycyclic molecules from acyclic precursors via radical mediated- or transition metal-catalyzed cascade reactions. Chem. Rev. 1996;96:289–306. doi: 10.1021/cr9500186. [DOI] [PubMed] [Google Scholar]
- 25.Biemolt J, Ruijter E. Advances in palladium-catalyzed cascade cyclizations. Adv. Synth. Catal. 2018;360:3821–3871. [Google Scholar]
- 26.Hong F-L, Ye L-W. Transition metal-catalyzed tandem reactions of ynamides for divergent N-heterocycle synthesis. Acc. Chem. Res. 2020;53:2003–2019. doi: 10.1021/acs.accounts.0c00417. [DOI] [PubMed] [Google Scholar]
- 27.Seregin IV, Gevorgyan V. Gold-catalyzed 1,2-migration of silicon, tin, and germanium en route to C-2 substituted fused pyrrole-containing heterocycles. J. Am. Chem. Soc. 2006;128:12050–12051. doi: 10.1021/ja063278l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith CR, Bunnelle EM, Rhodes AJ, Sarpong R. Pt-catalyzed cyclization/1,2-migration for the synthesis of indolizines, pyrrolones, and indolizinones. Org. Lett. 2007;9:1169–1171. doi: 10.1021/ol0701971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yan B, Zhou Y, Zhang H, Chen J, Liu Y. Highly efficient synthesis of functionalized indolizines and indolizinones by copper-catalyzed cycloisomerizations of propargylic pyridines. J. Org. Chem. 2007;72:7783–7786. doi: 10.1021/jo070983j. [DOI] [PubMed] [Google Scholar]
- 30.Seregin IV, Schammel AW, Gevorgyan V. Base- and ligand-free room-temperature synthesis of N-fused heteroaromatic compounds via the transition metal-catalyzed cycloisomerization protocol. Org. Lett. 2007;9:3433–3436. doi: 10.1021/ol701464j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seregin IV, Schammel AW, Gevorgyan V. Multisubstituted N-fused heterocycles via transition metal-catalyzed cycloisomerization protocols. Tetrahedron. 2008;64:6876–6833. doi: 10.1016/j.tet.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bunnelle EM, et al. Pt-catalyzed cyclization/migration of propargylic alcohols for the synthesis of 3(2H)-furanones, pyrrolones, indolizines, and indolizinones. Tetrahedron. 2008;64:7008–7014. doi: 10.1016/j.tet.2008.02.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chernyak D, Skontos C, Gevorgyan V. Two-component approach toward a fully substituted N-fused pyrrole ring. Org. Lett. 2010;12:3242–3245. doi: 10.1021/ol1011949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chernyak D, Gevorgyan V. Palladium-catalyzed intramolecular carbopalladation/cyclization cascade: access to polycyclic N-fused heterocycles. Org. Lett. 2010;12:5558–5560. doi: 10.1021/ol102447s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li Z, Chernyak D, Gevorgyan V. Palladium-catalyzed carbonylative cyclization/arylation cascade for 2‑aroylindolizine synthesis. Org. Lett. 2012;14:6056–6059. doi: 10.1021/ol302947r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aggarwal T, et al. Site-selective electrophilic cyclization and subsequent ring-opening: a synthetic route to pyrrolo[1,2‑a] quinolines and indolizines. J. Org. Chem. 2012;77:8562–8573. doi: 10.1021/jo3015374. [DOI] [PubMed] [Google Scholar]
- 37.Xu T, Alper H. Synthesis of indolizine derivatives by Pd-catalyzed oxidative carbonylation. Org. Lett. 2015;17:4526–4529. doi: 10.1021/acs.orglett.5b02220. [DOI] [PubMed] [Google Scholar]
- 38.Oh KH, Kim SM, Park SY, Park JK. Base-controlled Cu-catalyzed tandem cyclization/alkynylation for the synthesis of indolizines. Org. Lett. 2016;18:2204–2207. doi: 10.1021/acs.orglett.6b00821. [DOI] [PubMed] [Google Scholar]
- 39.Goulart TAC, Back DF, Zeni G. Copper-catalyzed carbon-nitrogen/carbon-selenium bonds formation: synthesis of 2-(organochalcogenyl)-indolizines. Adv. Synth. Catal. 2017;359:1901–1911. [Google Scholar]
- 40.Li Y, Xiong W, Zhang Z, Xu T. Synthesis of indolizine derivatives triggered by the oxidative addition of aroyl chloride to Pd (0) complex. J. Org. Chem. 2020;85:6392–6399. doi: 10.1021/acs.joc.0c00161. [DOI] [PubMed] [Google Scholar]
- 41.Ma J, Zhang L, Zhu S. Enynal/Enynone: a safe and practical carbenoid precursor. Curr. Org. Chem. 2016;20:102–118. [Google Scholar]
- 42.Chen L, Chen K, Zhu S. Transition-metal-catalyzed intramolecular nucleophilic addition of carbonyl groups to alkynes. Chem. 2018;4:1208–1262. [Google Scholar]
- 43.Zhu D, Chen L, Fan H, Yao Q, Zhu S. Recent progress on donor and donor–donor carbenes. Chem. Soc. Rev. 2020;49:908–950. doi: 10.1039/c9cs00542k. [DOI] [PubMed] [Google Scholar]
- 44.Woodward RB, Hoffmann R. Stereochemistry of electrocyclic reactions. J. Am. Chem. Soc. 1965;87:395–397. [Google Scholar]
- 45.Woodward RB, Hoffmann R. The conservation of orbital symmetry. Angew. Chem. Int. Ed. Engl. 1969;8:781–932. [Google Scholar]
- 46.Li Y, et al. Copper and rhodium relay catalysis for selective access to cis-2,3-dihydroazepines. Org. Lett. 2021;23:6450–6454. doi: 10.1021/acs.orglett.1c02262. [DOI] [PubMed] [Google Scholar]
- 47.Luo H, et al. Divergent synthesis of highly substituted pyridines and benzenes from dienals, alkynes, and sulfonyl azides. Org. Lett. 2021;23:7883–7887. doi: 10.1021/acs.orglett.1c02900. [DOI] [PubMed] [Google Scholar]
- 48.Du L, et al. Cascade 8π electrocyclization/benzannulation to access highly substituted phenylpyridines. Org. Lett. 2021;23:7966–7971. doi: 10.1021/acs.orglett.1c02968. [DOI] [PubMed] [Google Scholar]
- 49.Liu L, Du L, Li B. Recent advances in 8π electrocyclization reactions. Chem. Commun. 2023;59:670–687. doi: 10.1039/d2cc04805a. [DOI] [PubMed] [Google Scholar]
- 50.Zhang L, Sun J, Kozmin SA. Gold and platinum catalysis of enyne cycloisomerization. Adv. Synth. Catal. 2006;348:2271–2296. [Google Scholar]
- 51.Corma A, Leyva-Pérez A, Sabater MJ. Gold-catalyzed carbon−heteroatom bond-forming reactions. Chem. Rev. 2011;111:1657–1712. doi: 10.1021/cr100414u. [DOI] [PubMed] [Google Scholar]
- 52.Fang G, Bi X. Silver-catalysed reactions of alkynes: recent advances. Chem. Soc. Rev. 2015;44:8124–8173. doi: 10.1039/c5cs00027k. [DOI] [PubMed] [Google Scholar]
- 53.Yoo K, et al. Recent organic transformations with silver carbonate as a key external base and oxidant. Catalysts. 2019;9:1032. [Google Scholar]
- 54.Yang J-M, et al. Catalytic B–H bond insertion reactions using alkynes as carbene precursors. J. Am. Chem. Soc. 2017;139:3784–3789. doi: 10.1021/jacs.6b13168. [DOI] [PubMed] [Google Scholar]
- 55.Qiu S, Chen L, Jiang H, Zhu S. CuCl/Et3N-catalyzed synthesis of indanone-fused 2-methylene pyrrolidines from enynals and propargylamines. Org. Lett. 2017;19:4540–4543. doi: 10.1021/acs.orglett.7b02121. [DOI] [PubMed] [Google Scholar]
- 56.Tan T-D, et al. Copper-catalyzed cascade cyclization of indolyl homopropargyl amides: stereospecific construction of bridged Aza-[n.2.1] skeletons. Angew. Chem. Int. Ed. 2019;58:9632–9639. doi: 10.1002/anie.201904698. [DOI] [PubMed] [Google Scholar]
- 57.Wong HNC, et al. Use of cyclopropanes and their derivatives in organic synthesis. Chem. Rev. 1989;89:165–198. [Google Scholar]
- 58.Cohen Y, Cohen A, Marek I. Creating stereocenters within acyclic systems by C–C bond cleavage of cyclopropanes. Chem. Rev. 2021;121:140–161. doi: 10.1021/acs.chemrev.0c00167. [DOI] [PubMed] [Google Scholar]
- 59.Moir M, Danon JJ, Reekie TA, Kassiou M. An overview of late-stage functionalization in today’s drug discovery. Expert Opin. Drug Discov. 2019;14:1137–1149. doi: 10.1080/17460441.2019.1653850. [DOI] [PubMed] [Google Scholar]
- 60.Guillemard L, Kaplaneris N, Ackermann L, Johansson MJ. Late-stage C–H functionalization offers new opportunities in drug discovery. Nat. Rev. Chem. 2021;5:522–545. doi: 10.1038/s41570-021-00300-6. [DOI] [PubMed] [Google Scholar]
- 61.Denisov ET, Tumanov VE. Estimation of the bond dissociation energies from the kinetic characteristics of liquid-phase radical reactions. Russ. Chem. Rev. 2005;74:825–858. [Google Scholar]
- 62.Tumanov VE, Kromkin EA, Denisov ET. Estimation of dissociation energies of C–H bonds in oxygen-containing compounds from kinetic data for radical abstraction reactions. Russ. Chem. Bull. 2002;51:1641–1650. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that all data generated in this study are available within the paper and its Supplementary Information files. The X-ray crystallographic coordinates for structures reported in this article have been deposited at the Cambridge Crystallographic Data Center (2a: CCDC 2141700; 2z: CCDC 2141701; 3a: CCDC 2239536; 4 m: CCDC 2141703). These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif. Any additional detail can be requested from the corresponding authors.