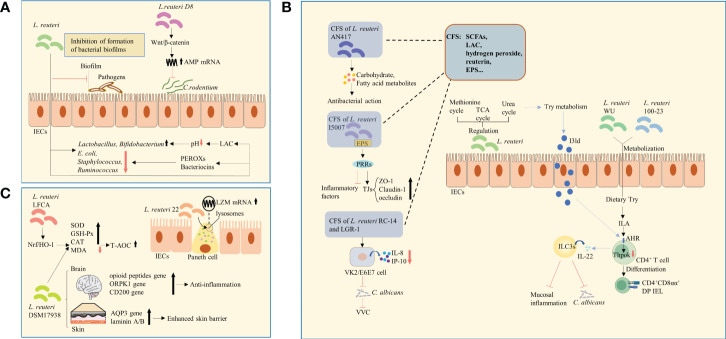
Figure 1.
Changes in microbial structure, metabolites, and expression of functional genes. (A) L. reuteri regulated the relative abundance of microorganisms. The antibacterial effect of L. reuteri is achieved through the production of LAC, which regulates the local pH and facilitates the growth of beneficial bacteria, or through the production of PEROXs and bacteriocins. L. reuteri activated the Wnt/β-catenin pathway, leading to increased expression of AMPs, thus inhibiting the colonization of C. rodentium. E.coli, Escherichia coli; LAC, lactic acid; PEROXs, peroxides; AMPs, antimicrobial peptides. (B) L. reuteri increased the levels of amino acid metabolites by regulating the urea, TCA, and methionine cycles, thus enhancing Try metabolism and in turn the anti-inflammatory ability. L. reuteri metabolized dietary Try to produce IAld, which acts as a ligand for the activation of the AHR on the surface of CD4+ T-cells, and induces ILC3s to produce IL-22. This process enhanced host resistance to C. albicans and protected against mucosal inflammation. Similarly, through the activation of Try metabolism and production of ILA that activate AHR and downregulate Thpok, L. reuteri promoted the differentiation of CD4+ T-cells into CD4+CD8αα+ DP IELs. The CSF of L. reuteri includes some of its metabolites and secreted bioactive factors, such as SCFAs, organic acids (such as LAC), hydrogen peroxide, bacteriocin compounds (such as reuterin), and EPS. The carbohydrate and fatty acid metabolites in CFS of L. reuturi AN417 has antibacterial action against oral pathogens. Binding of EPS of L. reuteri I5007 to PRRs was suggested to downregulate inflammatory factors and upregulate claudin-1, occludin, and ZO-1, thus enhancing intestinal barrier function. The CFS of L. reuteri RC-14 combined with that of LGR-1 inhibited the secretion of IL-8 and IP-10. It was shown to inhibit the colonization and growth of C. albicans and occurrence of VVC. TCA, tricarboxylic acid; Try, tryptophan; IAld, indole-3-aldehyde; AHR, aryl hydrocarbon receptor; ILC3s, group 3 innate lymphoid cells; IL-22, interleukin-22; C. albicans, Candida albicans; ILA, indole lactic acid; DP IELs, double-positive intraepithelial lymphocytes; CFS, Cell-free supernatant; EPS, exopolysaccharide; PRRs, pattern recognition receptors; ZO-1, zonula occludens 1; LGR-1, Lacticaseibacillus rhamnosus GR-1; IP-10, interferon-inducible protein-10; VVC, vulvovaginal candidiasis; SCFAs, short-chain fatty acids; LAC, lactic acid. (C) L. reuteri 22 promoted the mRNA expression of LZM and enhanced congenital immunity of intestinal mucosa. L. reuteri DSM 17938 increased the expression of skin epidermal AQP3 and lamininA/B, which strengthens skin barrier function. It also upregulated the expression of genes of opioid peptide, OPRK1, and CD200, which are related to stress and pain in the brain and are involved in anti-inflammatory signaling pathways. L. reuteri -LFCA improved oxidative stress-related indicators by activating Nrf2/HO-1 signaling pathway. The level of MDA was reduced, whereas those of enzymes, such as SOD, GSH-Px, and catalase were increased. L. reuteri DSM 17938 had similar effects. LZM, lysozyme; AQP3, aquaporin 3; kappa-opioid receptor 1, OPRK1; Nrf2/HO-1, nuclear factor E2-related factor 2/Heme oxygenase1; MDA, malondialdehyde; SOD, superoxide dismutase; GSH-Px, glutathione peroxidase.