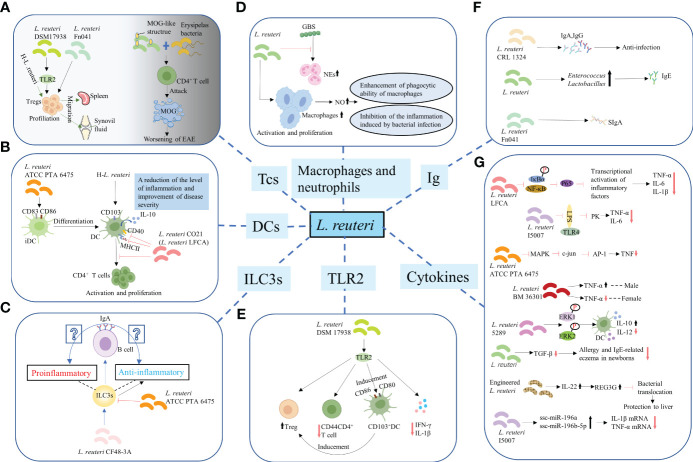
Figure 2.
Regulation of the differentiation and function of immune cells. (A) T-cells. L. reuteri 17938 and L. reuteri Fn041 stimulated the production of Foxp3+ Treg cells. Similarly, h-L.reuteri increased the numbers of Treg cells in the spleen and joint drainage lymph nodes. L. reuteri has a structure similar to that of the mouse MOG. It cooperated with erysipelas bacteria to activate CD4+ T-cells, which have the ability to attack MOG, leading to worsening of EAE. h-L. reuteri, heat-killed L. reuteri; MOG, myelin oligodendrocyte glycoprotein; EAE, experimental autoimmune encephalomyelitis. (B) Dendritic cells. L. reuturi ATTC PTA 6475 and its secreted factors induced the expression of CD83 and CD86 on the surface of iDCs and promoted the production of IL-10 by DCs. L. reuteri CO21 inhibited the expression of CD40 and MHCII on the surface of DCs and the activation and proliferation of CD4 + T-cells induced by DCs, thus achieving an anti-inflammatory effect. H-L.reuteri increased the numbers of CD103 + DCs, resulting in a reduction of the level of inflammation and improvement of disease severity. iDCs, immature dendritic cells; IL-10, interleukin-10; DCs, dendritic cells. (C) ILC3s. L. reuteri ATCC PTA 6475 reduced the number of ILC3s. L. reuteri CF48-3A promoted the production of ILC3s and induced the differentiation of IgA+ B–cells, leading to the generation of IgA. However, whether the IgA play a protective role against infection and enhances mucosal immunity or induces autoimmune effects remains unclear. ILC3s, Group 3 innate lymphoid cells. (D) Macrophages and NEs. L. reuteri increased production of NO by activated and increased numbers of macrophages, enhancing their phagocytotic ability. Meanwhile, L. reuteri inhibited the inflammation induced by bacterial infection by regulating the levels of NO. However, L. reuteri reduced the GBS-induced proliferation of NEs. NEs, neutrophils; NO, nitric oxide; GBS, Group B Streptococcus. (E) TLR2. L. reuteri 17938 is a bacterium that following recognition by TLR2 mediates anti-inflammatory immune response in the following ways: First, it led to an increase in the number of Foxp3+ Treg cells and a decrease in that of CD4+CD44+ Teffs. Second, it induced the generation and activation of CD103+ DCs, which were characterized by the expression of co-stimulative markers CD80 and CD86. This DC-induced production of Foxp3+ Treg cells is crucial for maintaining intestinal immune tolerance. Finally, activation of TLR2 mediated the decrease in the levels of IL-1β and IFN-γ.TLR2, Toll-like receptor 2; Teffs, effector T-cells; IFN-γ, interferon-γ. (F) Promotion of synthesis and secretion of Ig. L. reuteri FN041 increased the synthesis of sIgA. L. reuteri CRL1324 not only stimulated the production of IgA, but also that of IgG, which is related to anti-infection functions. An increase in the numbers of lactobacilli and enterococci caused by administration of mixed strains of L. reuteri were correlated with increased levels of IgE. Ig, Immunoglobulin; sIgA, secreted IgA. (G) Regulation of cytokines for improving intestinal mucosal barrier structure and permeability.