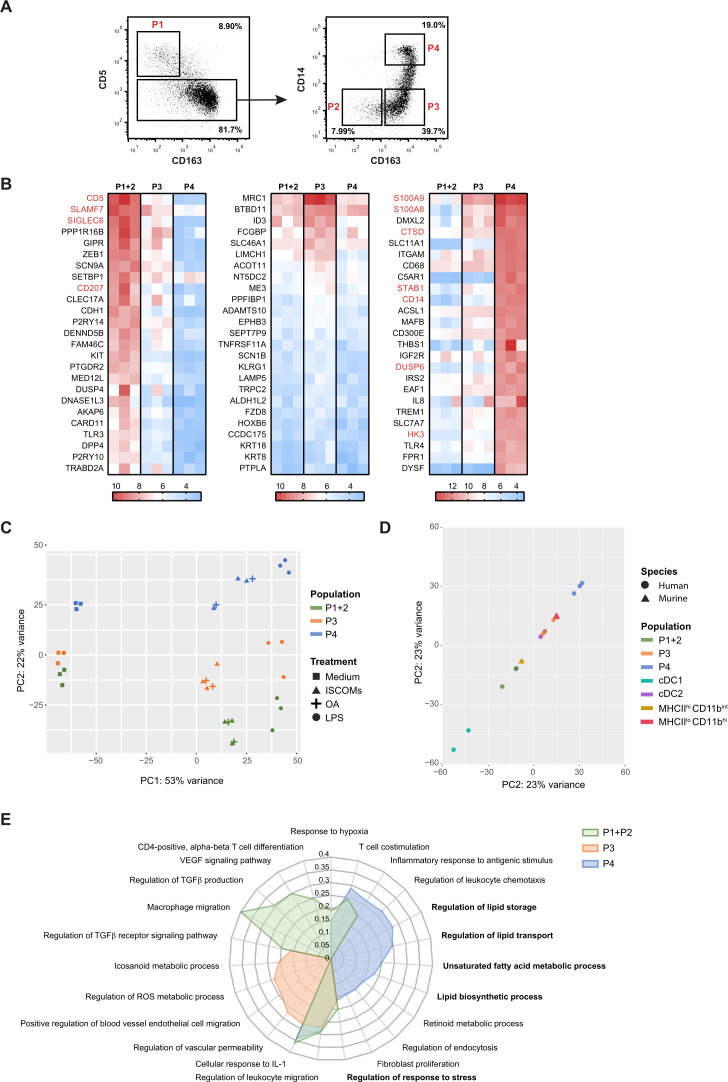
Figure 4.
ISCOMs upregulate lipid-metabolism-mediated pathways in CD163+ CD14+ cDC2s. (A) Gating strategy of the CD11c+ CD1c+ cDC2 subsets: P1 (CD5+), P2 (CD5− CD163−), P3 (CD5− CD163+ CD14−), and P4 (CD5− CD163+ CD14+) by flow cytometry. (B) Top 25 highest expressed genes for cDC2 subsets P1+P2 (left heatmap), P3 (middle heatmap), and P4 (right heatmap) without ISCOMs stimulation based on transformed normalized counts from RNA sequencing. Known genes for each subset according to literature are depicted in red. (C) PCA for bulk-sequenced P1+2, P3, and P4 cDC2s before and after 8 hours of ISCOMs, LPS, or OA stimulation. (D) PCA of bulk-sequenced human cDC1, cDC2, P1+2, P3, P4 after 8 hour ISCOM stimulation and murine MHCIIhi CD11bint and MHCIIlo CD11bhi DCs after 5 hours of ISCOMs stimulation. (E) GO enrichment for Biological Processes based on DEGs with p<0.05 and fold change ≥2 after stimulation with ISCOMs for 8 hours. Immunologically relevant GO pathways were selected and displayed as a radar plot showing the gene ratio. RNA sequencing for bulk cDC1s and cDC2s was performed on two healthy donors, for cDC2 subsets on three healthy donors, and for the murine subsets from two mice. cDC1, conventional type 1 DC; cDC2, conventional type 2 DC; DEGs, differentially expressed genes; GO, gene ontology; IL, interleukin; ISCOMs, immune stimulatory complexes; LPS, lipopolysaccharide; MHC, major histocompatibility complex; OA, oleic acid; PCA, principal component analysis; ROS, reactive oxygen species; TGF, transforming growth factor; VEGF, vascular endothelial growth factor.