Abstract
Background:
African Americans (AAs) experience higher rates of preterm birth and fetal growth restriction relative to other pregnant populations. Differential in utero exposure to environmental chemicals may partially explain these health disparities, as AAs are disproportionately exposed to environmental hazards.
Objective:
We examined the individual and mixture effects of non-persistent chemicals and persistent organic pollutants (POPs) on gestational age at birth and birthweight for gestational age z-scores within a prospective cohort of pregnant AAs.
Methods:
First-trimester serum and urine samples obtained from participants within the Atlanta African American Maternal-Child cohort were analyzed for 43 environmental chemicals, including per-and polyfluoroalkyl substances (PFAS), polybrominated diphenyl ethers (PBDEs), organochlorine pesticides, pyrethroid insecticides, phthalates, bisphenol A, nicotine, and the primary metabolite of delta-9-tetrahydrocannabinol. Linear regression was used to estimate individual associations between chemicals and gestational age and birthweight z-scores (N ranging from 107 to 523). Mixture associations were estimated using quantile g-computation, principal component (PC) analyses, and hierarchical Bayesian kernel machine regression among complete cases (N=86).
Results:
Using quantile g-computation, increasing all chemical exposures by one quantile was modestly associated with a reduction in gestational age (mean change per quartile increase= −0.47, 95% CI= −1.56, 0.61) and birthweight z-scores (mean change per quartile increase= −0.49, 95% CI= −1.14, 0.15). All PCs were associated with a reduction in birthweight z-scores; associations were greatest in magnitude for the two PCs reflecting exposure to combined tobacco, insecticides, PBDEs, and phthalates. In single pollutant models, we observed inconsistent and largely non-significant associations.
Signifance:
We conducted multiple targeted exposure assessment methods to quantify levels of environmental chemicals and leveraged mixture methods to quantify their joint effects on gestational age and birthweight z-scores. Our findings suggest that prenatal exposure to multiple classes of persistent and non-persistent chemicals is associated with reduced gestational age and birthweight z-scores in AAs.
Keywords: pregnancy, mixtures, exposure assessment, disparities
Introduction
Low birthweight and preterm birth are the leading causes of infant morbidity and mortality worldwide.1,2 Disparities in adverse pregnancy outcomes are well-documented, and African Americans (AAs) consistently experience preterm birth and fetal growth restriction at rates nearly double their white counterparts.3 Known risk factors for adverse pregnancy outcomes include older maternal age, smoking or alcohol consumption during pregnancy, pre-existing conditions (e.g., obesity, hypertension, and diabetes), and lower socioeconomic status (SES). However, these known risk factors do not fully account for health disparities in pregnancy and birth outcomes,4 suggesting that environmental exposures, which disproportionately impact AAs, may be contributing factors.5-7
Over 350,000 chemicals and their mixtures are registered for use in commerce.8 However, the actual number of chemicals on the global market is believed to be substantially higher.8 Only a small fraction of these chemicals have been tested for human health effects; even fewer are routinely included in human biomonitoring studies. This is problematic, as representative studies find that nearly all individuals in the United States have detectable levels of multiple environmental chemicals.9-11
Some of the most widely detected chemicals in our environment include non-persistent and persistent organic pollutants (POPs). These chemicals are found in food and water sources, personal care products, home furnishings, and elsewhere.12,13 POPs include several per-and polyfluoroalkyl substances (PFAS), polybrominated diphenyl ethers (PBDEs), and organochlorine pesticides (OCPs), while examples of non-persistent chemicals include pyrethroid and organophosphate insecticides, phthalates and alkylated phenols. A defining characteristic of POPs is that they do not easily break down in the environment and body, indicating that exposures can persist for many years, regardless of whether the compound is removed from commerce.12,14,15 In contrast, non-persistent chemicals have a short half-life of only a few hours or days and are readily excreted in urine.16 Despite this, human biomonitoring studies show that exposure to both persistent and non-persistent chemicals is ubiquitous due to continuous exposures.17-20
Exposure to environmental chemicals is particularly troubling during pregnancy, as many have been linked to adverse pregnancy outcomes.21-24 Additionally, these chemicals have been detected in the cord blood of newborns,19,25,26 indicating they can cross the placenta and directly expose the developing fetus. Despite this, our understanding of their effects on perinatal health largely comes from studies that have examined a single chemical at a time. Historically, studies that have examined mixtures of chemicals have largely focused on a single chemical class,21,27-29 which does not account for exposure to numerous chemicals simultaneously.17-19
To date, we have a limited understanding of how cumulative exposure to environmental chemicals influences adverse pregnancy outcomes.30-32 This is critical, as individual chemicals across classes may work together to produce additive or synergistic effects. Furthermore, few studies have been conducted among AAs, a historically marginalized population that consistently experiences elevated rates of adverse pregnancy outcomes and is often exposed to the highest levels of environmental hazards.
Notably, environmental exposures are highly plausible and potentially major contributors to racial disparities in adverse pregnancy outcomes,4 as racial residential segregation, a tactic historically known as redlining, has had a powerful impact on psychosocial and physical exposures that disproportionately impacts Black Americans.33 Additionally, systematic racism that has resulted in the isolation and disenfranchisement of predominantly Black communities, continues to facilitate residential segregation and unequal distribution of resources between Black and white communities.33 In fact, sites that emit toxicants most hazardous to human health, such as landfills and chemical plants, are disproportionately placed in non-white communities.34 Despite our knowledge of this history of calculated disenfranchisement, we have yet to fully understand the biological basis of health inequities that are made manifest through our nation’s history of civic and environmental injustice.
To address this knowledge gap, we leveraged the Atlanta African American Maternal-Child cohort,35,36 which has previously quantified levels of 43 emerging parent and/or metabolites of selected environmental chemicals, including PFAS, PBDEs, OCPs, pyrethroid and organophosphate insecticides, phthalates, bisphenol A (BPA), nicotine, and the primary metabolite of delta-9-tetrahydrocannabinol, a cannabinoid molecule in marijuana, during early pregnancy. To better understand how these chemicals influence fetal development, we utilized three different mixtures approaches to estimate the associations between individual chemicals and their joint effects on gestational age at birth and birthweight for gestational age z-scores, a proxy for fetal growth. We hypothesized that an increase in the combined exposure levels would be associated with a reduction in gestational age and birthweight z-scores.
2. Methods
2.1. Study Population
Our analytic sample included 547 participants enrolled between 2014-2019 in the Atlanta African American Maternal-Child Cohort, an ongoing prospective birth cohort described in detail elsewhere.35,36 Briefly, pregnant women were recruited between 8-14 weeks gestation from two hospitals in metropolitan Atlanta, Georgia. Participants recruited from Emory Healthcare are generally higher SES, while those recruited from Grady Health Systems are more socioeconomically diverse. As part of the study, participants consented to a review of their medical record and provided blood and urine during early pregnancy (range: 8-14 weeks gestation). Individuals were eligible for inclusion if they self-identified as AA and female, not pregnant with multiples, fluent in English, and had no chronic medical conditions. All participants provided written, informed consent prior to participating. The Institutional Review Board at Emory University approved the ATL AA study (approval reference number 68441).
2.2. Environmental Chemical Exposure Assessment
Concentrations of phthalates, BPA, pyrethroid insecticides, cotinine, and 11-nor-9-carboxy-Δ9-tetrahydrocannabinol (COOH-THC; the main psychoactive constituent of marijuana) were analyzed in urine, and concentrations of PFAS, PBDEs, and OCPs were analyzed in serum obtained between 8-14 weeks gestation. Serum and urine samples were stored at −80°C prior to analysis. Across all chemicals, values below the limit of detection (LOD), the concentration was replaced with LOD/. We natural log transformed all chemicals for downstream analyses, as all distributions were right skewed.37
Urinary creatinine was measured in the 1000-fold diluted urine samples without extraction. The diluted urine samples were spiked with the stable isotope analog, injected, and analyzed using an LC-MS/MS instrument operated in negative electrospray ionization mode.38 The target compound was analyzed using multi-reaction monitoring mode. Quantification of urinary creatinine was performed using isotope dilution calibration. A matrix-matched standard calibration curve was used. Replicates of NIST SRM 3667 were included in the analysis and the recoveries were well within ±20 of the certified values.
Across chemical classes, the sample size ranged from 107 to 523, as chemical exposure assessment was conducted across numerous projects and was limited by funding. There were 86 participants who had serum and urine sample analyzed for all environmental chemicals. Thus, we conducted our mixture analyses on those chemicals with >70% above the LOD within the complete cases (N=86). This included 4 PFAS (PFHxS, PFOA, PFOS, PFNA), 2 OCPs (HCB, p’p’-DDE), 2 PBDEs (BDE-100, BDE-47), one insecticide (3-PBA), 7 phthalates (MEP, MBP, MiBP, MBzP, MEHP, MEOHP, MEHHP), 2 biomarkers of tobacco exposure (3OH-COT, COT), and BPA.
2.2.1. Per- and poly-fluoroalkyl substances (PFAS)
Serum samples were analyzed for PFAS levels at the Children’s Health Exposure Analysis Resource (CHEAR) and Human Health Exposure Analysis Resource (HHEAR) laboratories, including Wadsworth Center/New York University Laboratory Hub (Wadsworth/NYU) and the Laboratory of Exposure Assessment and Development for Environmental Research (LEADER) at Emory University. Laboratories in CHEAR and HHEAR have participated in activities to produce harmonized measurements among them.39 The details of analytical methods used in both labs have been described in detail elsewhere.6,40 Briefly, each serum sample was spiked with isotopic internal standards, treated by solid phase extraction, and analyzed using a liquid chromatographic-tandem mass spectrometric (LC-MS/MS) instrument operated in negative electrospray ionization mode. The target compounds were analyzed using multi-reaction monitoring mode. Quantification of target PFAS was performed using isotope dilution calibration. A matrix-matched standard calibration curve was used. Bench and blind quality control samples and blanks were analyzed alongside unknown samples. Replicates of standard reference materials (SRM) from National Institute of Standards and Technology (NIST) (SRM 1958) were included in the analysis to ensure the quality of the data produced. The recoveries of the NIST SRM materials were well within the acceptable range (i.e., ±20 of the certified values). Both laboratories participate in and are certified by the German External Quality Assessment Scheme twice annually for serum PFAS quantification and laboratory measurements have been cross-validated between the two labs conducting PFAS measurements (the Pearson correlation coefficients ranged from 0.88 to 0.93, and the relative percent differences ranged from 0.12 to 20.2% with a median of 4.8% in the 11 overlapping samples).6
2.2.2. Polybrominated diphenyl ethers (PBDEs)
Concentrations of PBDE congeners BDE–47, BDE–85, BDE–99, BDE–100, BDE–153, and BDE–154 were analyzed in the LEADER laboratory at Emory University. This method has been described in detail elsewhere.41-43 Briefly, samples were fortified with isotopically labeled analogues of the target chemicals, homogenized and deprotonated. Supernatants subsequently were extracted twice with hexane and dichloromethane and passed through an activated silica gel column to remove residual biogenic material. Sample extracts were concentrated, injected, and analyzed using a gas chromatographic-tandem mass spectrometric (GC-MS/MS) instrument. The target compounds were analyzed using multi-reaction monitoring mode. Quantification of the target PBDEs was performed using isotope dilution calibration. A solvent-based standard calibration curve was used. Replicates of NIST SRM 1958 were included in the analysis and the recoveries were well within ±20 of the certified values. We did not adjust for total lipids, as information on maternal serum total cholesterol and free triglycerides was not available in our analytic sample.
2.2.3. Organochlorine pesticides (OCPs)
Analysis of OCPs was conducted at the LEADER laboratory using a modified version of the method described by Marder et al.44 OCPs included hexachlorobenzene (HCB), β-hexachlorocyclohexane (β-HCH), 1,1-dichloro-2,2-bis(4- 4- chlorophenyl )ethylene (o,p’-DDE), transnonachlor (TNC), dichlorodiphenyldichloroethylene (p,p’-DDE), 1,1,1-tricloro-2,2-bis (p-chlorophenyl) ethane (p,p’-DDT) o,p'-1,1'-(2,2,2-Trichloroethane-1,1-diyl)bis(4- chlorobenzene) (o,p’-DDT), and 3,5,6-trichloro-2-pyridinol (TCPy). Briefly, serum samples were fortified with isotopically labeled analogues of the target chemicals and subjected to liquid–liquid extraction followed by solid-phase extraction. Sample extracts were concentrated, injected, and analyzed using a GC-MS/MS instrument. The target compounds were analyzed using multi-reaction monitoring mode. Quantification of the target OCPs was performed using isotope dilution calibration. A matrix-matched standard calibration curve was used. Replicates of NIST SRM 1958 were included in the analysis and the recoveries were well within ±20 of the certified values.44
2.2.4. Pyrethroid insecticides
Urine samples were analyzed for a common metabolite of synthetic pyrethroids, 3-phenoxybenzoic acid (3-PBA), and two specific metabolites of permethrin, cypermethrin and cyfluthrin, cis- or trans-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropane carboxylic acid (cis-DCCA, trans-DCCA), using a modification of a previously validated method.45 Briefly, samples were spiked with stable isotopic analogues of the target analytes and then enzymatically digested using purified β-glucuronidase and sulfatase enzymes (derived from H. pomatia) to liberate bound metabolites. The hydrolyzed urine samples were extracted using solid phase extraction. Sample extracts were concentrated, injected, and analyzed using an LC-MS/MS instrument operated in negative electrospray ionization mode. The target compounds were analyzed using multi-reaction monitoring mode. Quantification of the target metabolites was performed using isotope dilution calibration. A matrix-matched standard calibration curve was used. The laboratory successfully participated in the proficiency testing program offered by the German External Quality Assessment Scheme (G-EQUAS).
2.2.5. Phthalates
Concentrations of eight maternal urinary phthalate metabolites were analyzed at the LEADER laboratory and included monoethyl phthalate (MEP), mono-n-butyl phthalate (MBP), monoisobutyl phthalate (MiBP), Monobenzyl phthalate (MBzP), mono(2-ethlyhexyl) phthalate (MEHP), mono(2-ethyl-5-oxohexyl) phthalate (MEOHP), mono (2-ethyl-5-hydroxyhexyl) phthalate (MEHHP), and mono(2-ethly-5-carboxypentyl) phthalate (MECPP). Measurement of phthalate metabolites has been previously described.46 Briefly, samples were spiked with stable isotopic analogues of the target analytes and then enzymatically digested using purified β-glucuronidase and sulfatase enzymes (derived from H. pomatia) to liberate bound metabolites. The hydrolyzed urine samples were extracted using solid phase extraction. Sample extracts were concentrated, injected, and analyzed using an LC-MS/MS instrument operated in negative electrospray ionization mode. The target compounds were analyzed using multi-reaction monitoring mode. Quantification of the phthalate metabolites was performed using isotope dilution calibration. A matrix-matched standard calibration curve was used. Replicates of NIST SRM 3672 and 3673 were included in the analysis and the recoveries were well within ±20 of the certified values.
2.2.6. Bisphenol A (BPA)
Exposure assessment of BPA was conducted at the LEADER laboratory using a modification of the method by Zhou et al.47 Briefly, a 1-mL aliquot of urine was spiked with isotopically labeled analogues of BPA, and was then subjected to an enzyme hydrolysis to liberate glucuronide-bound conjugates. The hydrolysate was subsequently extracted using an ABS Elut-NEXUS solid phase extraction column, eluting with acetonitrile and ethyl acetate. The extract was concentrated to dryness and reconstituted in mobile phase for analysis using LC-MS/MS. Analyte concentrations were calculated using isotope dilution calibration. To avoid biases between batches, samples were randomized according to a Fisher-Yates algorithm.48,49
2.2.7. Tobacco and Marijuana
The LEADER laboratory measured concentrations of cotinine (COT), trans-3-hydroxycotinine (3OH-COT), and 11-nor-9-carboxy-Δ9-tetrahydrocannabinol (COOH-THC) using a fully validated method.50 COT and 3OH-COT are the primary metabolites of nicotine and often serve as biomarkers of tobacco exposure. COOH-THC is the primary metabolite of delta-9-tetrahydrocannabinol, the main psychoactive constituent of cannabis (marijuana), and COOH-THC concentrations reflect exposure to marijuana. Urine samples were spiked with stable isotopic analogues of the target analytes and then enzymatically digested using purified β-glucuronidase and sulfatase enzymes (derived from H. pomatia) to liberate bound metabolites. The hydrolyzed urine samples were extracted using supported liquid extraction. Sample extracts were concentrated, injected, and analyzed using an LC-MS/MS instrument. The instrument was operated in positive electrospray ionization mode during the analysis of COT and 3OH-COT and in negative mode for COOH-THC. The target compounds were analyzed using multi-reaction monitoring mode. Quantification of the target metabolites was performed using isotope dilution calibration. A matrix-matched standard calibration curve was used. Replicates of NIST SRM 3672, 3673, and 1507b were included in the analysis and the recoveries were well within ±20 of the certified values.
2.3. Birth Outcomes
Gestational age at delivery was abstracted from the medical record and estimated using best obstetrical estimate based upon the date of delivery in relation to the estimated date of confinement, as recommended by the American College of Obstetrics and Gynecology.51 Birthweight in grams was obtained from the first weight measured in the delivery room. As a proxy for fetal growth, we calculated sex-specific birthweight for gestational age z-scores using a US population-based reference for singleton births.52 Due to sample size limitations and to enhance statistical power, we focused our analysis on continuous outcomes and did not examine categorical definitions of preterm birth or small for gestational age.
2.4. Covariates
Marital status (married or partnered and co-habiting, single or partnered, and not cohabiting), maternal level of education (less than high school, high school diploma, college degree, graduate degree), type of health insurance during pregnancy (private, public), and number of members in the household were obtained via a standardized interview questionnaire that was administered at enrollment. An income to poverty ratio was calculated by using a combination of the number of members in the household and self-reported annual household income. Maternal body mass index (BMI; kg/m2) was calculated using weight and height measurements obtained during the first clinical visit between 8-14 weeks’ gestation. Information regarding parity and self-reported alcohol consumption during pregnancy was abstracted from the medical record.
2.5. Statistical Analysis
We examined the distributions of demographic characteristics in our analytic sample using means, standard deviations (SDs), frequencies, and counts. The distributions of chemicals were assessed using geometric means, geometric SDs, and selected percentiles. Correlations between chemicals with >70% detection were estimated using Spearman correlation coefficients (ρ). Unadjusted and adjusted linear regression models were used to estimate single pollutant association between chemicals and gestational age and birthweight z-scores, respectively. In these models, all chemicals were natural log transformed and standardized to the population’s interquartile range (IQR). Single pollutant associations were estimated among complete cases included in mixture models, as well as among the largest possible analytic sample for each chemical class. Covariates retained in adjusted models included maternal education, maternal age, parity, alcohol consumption, and early pregnancy body mass index (BMI). These covariates were chosen via a Directed Acyclic Graph (DAG; Figure S1) that was informed by a literature review and associations with exposures and outcomes in our study population.
We used three approaches to estimate the cumulative effect of exposures to all chemicals on birth outcomes. Mixtures analyses were restricted to complete cases that had information on all chemicals (N=86) and were adjusted for the same set of covariates as linear regression models. First, we utilized quantile g-computation, which estimates the effect of simultaneously increasing all exposures in the mixture by one quartile.53 With this method, all exposures included in the mixture are assigned a positive or negative weight, based on the direction of independent effect. Positive and negative weights sum to 1 and are interpreted as the proportion of the partial effect in the positive or negative direction due to a single exposure. Effect estimates obtained from quantile g-computation are interpreted as the effect on the outcome (birthweight z-scores or gestational age) associated with simultaneously increasing all chemical exposures in the mixture by one quantile.
Our second approach utilized principal component analysis (PCA), a data reduction technique that enables us to identify clusters of exposure patterns. Using scree plots, we identified five principal components (PCs) that explained 68% of the variance in our data. We then used linear regression to examine unadjusted and adjusted associations between the five PCs and gestational age and birthweight z-scores. All PCs were included concurrently in the same model, as PCA produces uncorrelated components without the worry of multicollinearity. Covariates retained in mixture models were analogous to those included in single analyte models.
We applied Bayesian Kernel Machine Regression (BKMR) with hierarchical variable selection in our third approach.54,55 BKMR was performed with 10,000 iterations and all chemicals were natural log transformed. We used BKMR with a hierarchical variable selection based on pre-defined groups of persistent (PFAS, PBDEs, OCPs, and pyrethroid insecticides) and non-persistent (tobacco, phthalates, and BPA) chemicals. We calculated the group posterior inclusion probability (groupPIP) and conditional posterior inclusion probability (condPIP), with the former representing the probability of including a particular biomarker group within the model and the latter representing the probability that a specific biomarker is included within its group. PIPs range from 0 to 1 and are used to highlight the top contributors to an observed mixture effect. We then assessed linearity by visually examining individual univariate exposure-response functions. We also estimated the overall mixture effect, which compares the change in the outcome when all chemicals are set at the 75th and 25th percentile relative to the median value. Lastly, we calculated individual effect estimates, which compares the effect on the outcome when each chemical is set at the 75th percentile relative to the 25th percentile holding all other chemicals constant.
We conducted a number of sensitivity analyses to examine the robustness of our findings. We first explored sex differences by examining single analyte associations stratified by infant sex. Second, because PBDEs are lipophilic and information on total lipid levels was unavailable in our analytic sample, we utilized quantile g-computation to estimate the mixture effect removing PBDEs (N=86). Third, we removed early pregnancy BMI from our linear regression models, as some chemicals included in our analyses are classified as obesogens.56 Lastly, in an effort to increase our sample size, we removed OCPs and PBDEs from quantile g-computation models (N=230).
3. Results
There were 547 participants who had information on gestational age and birthweight for gestational z-scores available at the time of our analysis. Of this group, 86 participants had biospecimens measured for PFAS, PBDES, OCPs, insecticides, phthalates, BPA, and marijuana and nicotine. In the full cohort, the mean maternal age was 25 years (SD=4.9) and mean early pregnancy BMI was 29 kg/m2 (SD=7.9) (Table 1). Approximately half of the participants were unmarried and not cohabitating (52%) and 68% had a high school or college degree. Relative to the full cohort, those retained in mixture models were more likely to be unmarried and not cohabitating (62%) and to have a high school degree (45%) (Table 1).
Table 1.
Demographic characteristics of Atlanta African American Maternal-Child study population, 2016-2020 and the sub-population used in this analysis.
| Full Cohort (N=547) | Analytic Sample for Mixtures (N=86) |
|
|---|---|---|
| N (%) or Mean (SD) | N (%) or Mean (SD) | |
| Maternal Age (years) | 25 (4.9) | 26 (4.7) |
| Maternal Body Mass Index (kg/m2) | 29 (7.9) | 29 (6.9) |
| Marital Status | ||
| Married/Co-habiting | 263 (48 %) | 33 (38 %) |
| Single | 284 (52 %) | 53 (62 %) |
| Maternal Education | ||
| <High School | 87 (16 %) | 13 (15 %) |
| High School | 213 (39 %) | 39 (45 %) |
| College Degree | 158 (29 %) | 21 (24 %) |
| Graduate Degree | 89 (16 %) | 13 (15 %) |
| Income to Poverty Ratio | ||
| <100% | 243 (44 %) | 39 (45 %) |
| 100-150% | 121 (22 %) | 16 (19 %) |
| 150-300% | 116 (21 %) | 16 (19 %) |
| >300% | 67 (12 %) | 15 (17 %) |
| Alcohol Use During Pregnancy | ||
| No | 483 (88 %) | 77 (90 %) |
| Yes | 64 (12 %) | 9 (10 %) |
| Parity | ||
| 0 | 254 (46 %) | 35 (41 %) |
| ≥1 | 293 (54 %) | 51 (59 %) |
| Health Insurance | ||
| Public | 433 (79 %) | 68 (79 %) |
| Private | 114 (21 %) | 18 (21 %) |
| Delivery Hospital | ||
| Emory | 221 (40 %) | 34 (40 %) |
| Grady | 326 (60 %) | 52 (60 %) |
| Infant Sex | ||
| Male | 257 (47 %) | 41 (48 %) |
| Female | 272 (50 %) | 45 (52 %) |
| Missing | 18 (3.3%) | 0 (0%) |
| Gestational Age (weeks) | 38 (4.6) | 39 (1.6) |
| Missing | 12 (2.2%) | 0 (0%) |
| Birthweight (grams) | 3026 (624) | 3062 (433) |
| Missing | 26 (4.8%) | 0 (0%) |
| Birthweight z-scores | −0.47 (1.1) | −0.44 (1.08) |
| Missing | 26 (4.8%) | 0 (0%) |
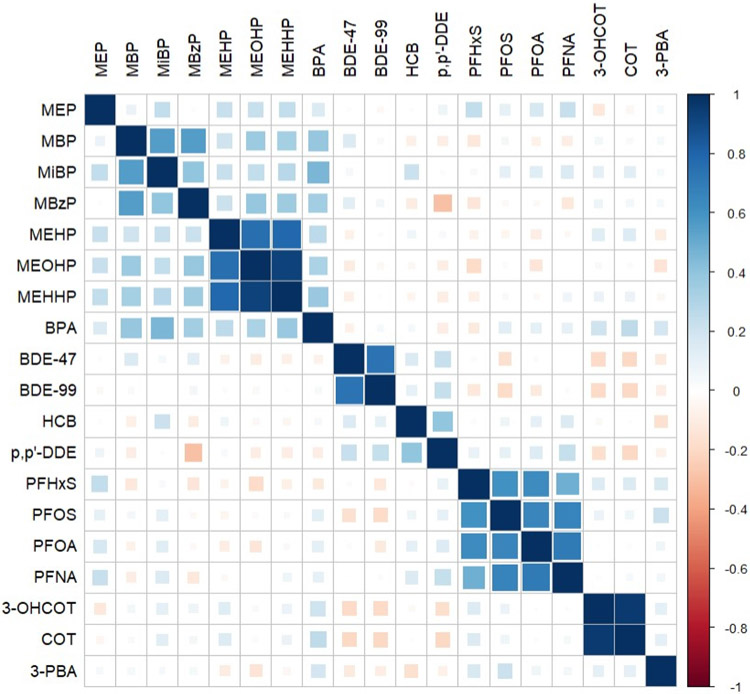
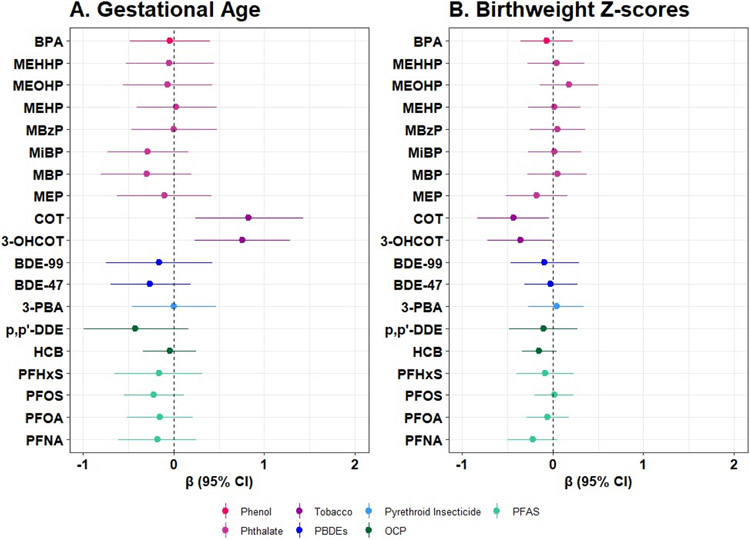
Among the PFAS, the highest geometric mean was for PFOS (geometric mean= 1.89 ng/mL, geometric SD= 2.05), while the highest geometric mean among the PBDEs and pyrethroid insecticides was for BDE-47 (geometric mean= 89.84 pg/mL, geometric SD= 2.08) and TCPy (geometric mean= 0.75 ng/mL, geometric SD= 4.15). Within the phthalate metabolites, MEP had the highest geometric mean (geometric mean= 0.65 μg/g creatinine, geometric SD= 2.83; Table 2). The distributions were similar when restricting to our analytic sample for mixture models (Table S1). Spearman correlation coefficients revealed that chemicals within a class were moderately to strongly correlated (Figure 1). With the exception of BPA and the phthalates, chemicals were not strongly correlated across classes. In adjusted single pollutant models restricted to our analytic sample for mixtures (N=86), we observed that COT and 3-OHCOT were associated with increased gestational age and reduced birthweight z-scores (Figure 2; Table S2). Individual biomarkers of PFAS, PBDEs, OCPs, pyrethroid insecticides, phthalates, and BPA were not strongly associated with gestational age and birthweight z-scores within this analytic sample (Table S2). In our linear regression models estimating single pollutant associations within the largest possible sample size, we observed that an IQR increase in BDE-99, BDE-47, ppDDE, HCB, and PFNA was associated with lower birthweight z-scores, although confidence intervals largely included the null value (Table S3). Associations were similar in linear regression models removing early pregnancy BMI (Table S4). When stratifying by infant sex, a non-significant inverse association between phthalate metabolite concentrations and gestational age was observed among males only (Table S5).
Table 2.
Distribution of first trimester serum and urinary levels of environmental chemicals in the Atlanta African American Maternal-Child study population, 2016-2020.
| Percentiles | ||||||||
|---|---|---|---|---|---|---|---|---|
| N | % Above LOD |
Geometric Mean (Geometric SD) |
5 | 25 | 50 | 75 | 95 | |
| Per-and polyfluoroalkyl substances (PFAS) (ng/mL) | ||||||||
| PFHxS | 523 | 97.51 | 1.16 (2.06) | 0.30 | 0.81 | 1.24 | 1.75 | 3.61 |
| PFOS | 523 | 97.9 | 1.89 (2.50) | 0.53 | 1.38 | 2.12 | 3.23 | 5.44 |
| PFOA | 523 | 97.32 | 0.62 (2.36) | 0.11 | 0.45 | 0.70 | 1.06 | 1.69 |
| PFNA | 523 | 96.75 | 0.26 (2.35) | 0.05 | 0.17 | 0.30 | 0.47 | 0.81 |
| PFBS | 428 | 27.57 | 0.03 (3.48) | 0.01 | 0.01 | 0.01 | 0.03 | 0.44 |
| PFHPA | 428 | 19.63 | 0.04 (2.03) | 0.02 | 0.04 | 0.04 | 0.04 | 0.22 |
| PFDA | 428 | 57.24 | 0.07 (2.85) | 0.03 | 0.03 | 0.07 | 0.17 | 0.40 |
| PFUNDA | 428 | 51.17 | 0.04 (3.03) | 0.01 | 0.01 | 0.02 | 0.09 | 0.27 |
| PFDODA | 428 | 7.94 | 0.03 (1.71) | 0.01 | 0.03 | 0.03 | 0.03 | 0.06 |
| PFOSA | 428 | 3.04 | 0.01 (1.28) | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| NETFOSAA | 355 | 5.07 | 0.01 (1.32) | 0.01 | 0.01 | 0.01 | 0.01 | 0.02 |
| NMFOSAA | 428 | 52.57 | 0.04 (3.04) | 0.01 | 0.01 | 0.03 | 0.09 | 0.26 |
| PFPEA | 355 | 48.17 | 0.06 (2.00) | 0.04 | 0.04 | 0.04 | 0.11 | 0.22 |
| PFHXA | 428 | 9.58 | 0.04 (1.99) | 0.01 | 0.04 | 0.04 | 0.04 | 0.21 |
| PFDS | 75 | 0 | 0.01 (1.00) | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| Organochlorine pesticides (OCPs) (ng/mL) | ||||||||
| HCB | 107 | 98.13 | 0.07 (1.41) | 0.04 | 0.05 | 0.06 | 0.07 | 0.11 |
| p’p’-DDE | 107 | 100 | 0.2 (1.74) | 0.09 | 0.14 | 0.20 | 0.28 | 0.53 |
| β-HCH | 107 | 0 | 0.2 (1.19) | 0.19 | 0.19 | 0.20 | 0.20 | 0.22 |
| TCPy | 300 | 68.67 | 0.75 (4.15) | 0.09 | 0.09 | 1.13 | 2.14 | 4.58 |
| o,p’-DDE | 107 | 1.87 | 0.2 (1.34) | 0.19 | 0.19 | 0.20 | 0.20 | 0.22 |
| TNC | 107 | 35.51 | 0.12 (2.20) | 0.03 | 0.05 | 0.20 | 0.20 | 0.20 |
| o,p’-DDT | 107 | 1.87 | 0.2 (1.26) | 0.19 | 0.19 | 0.20 | 0.20 | 0.22 |
| p,p’-DDT | 107 | 0.93 | 0.2 (1.21) | 0.19 | 0.19 | 0.20 | 0.20 | 0.22 |
| Pyrethroid insecticides (μg/g creatnine) | ||||||||
| 3-PBA | 251 | 75.58 | 0.003 (4.90) | 0.002 | 0.002 | 0.004 | 0.01 | 0.03 |
| trans-DCCA | 251 | 10.56 | 0.01 (2.64) | 0.002 | 0.002 | 0.004 | 0.01 | 0.04 |
| cis-DCCA | 251 | 6.93 | 0.01 (2.41) | 0.002 | 0.003 | 0.004 | 0.01 | 0.03 |
| Polybrominated diphenyl ethers (PBDEs) (pg/mL) | ||||||||
| BDE-47 | 311 | 100 | 89.84 (2.08) | 32.9 | 55.52 | 83.86 | 136.46 | 352.58 |
| BDE-99 | 311 | 78.14 | 23.1 (2.35) | 7.81 | 10.91 | 22.11 | 38.19 | 104.89 |
| BDE-100 | 311 | 73.63 | 13.32 (2.99) | 2.81 | 3.29 | 15.44 | 31.3 | 70.36 |
| BDE-85 | 311 | 1.93 | 48.58 (2.05) | 16.67 | 17.86 | 78.12 | 78.12 | 78.12 |
| BDE-154 | 311 | 8.36 | 86.5 (1.24) | 78.12 | 78.12 | 78.12 | 89.29 | 130.41 |
| BDE-153 | 311 | 11.25 | 57.4 (1.96) | 16.67 | 51.63 | 78.12 | 78.12 | 125 |
| Marijuana and tobacco (μg/g creatnine) | ||||||||
| 3-OHCOT | 252 | 70.63 | 0.15 (17.38) | 0.004 | 0.01 | 0.11 | 1.29 | 24.00 |
| COT | 252 | 71.03 | 0.08 (13.39) | 0.003 | 0.01 | 0.05 | 0.65 | 9.96 |
| COOH-THC | 304 | 41.12 | 11.91 (5.68) | 3.5 | 3.5 | 3.5 | 42.79 | 432.46 |
| Phthalate metabolites (μg/g creatnine) | ||||||||
| MEP | 245 | 100 | 0.65 (2.83) | 0.14 | 0.3 | 0.57 | 1.31 | 4.41 |
| MBP | 245 | 83.67 | 0.06 (2.61) | 0.01 | 0.03 | 0.07 | 0.11 | 0.3 |
| MiBP | 245 | 85.71 | 0.05 (2.52) | 0.01 | 0.03 | 0.05 | 0.1 | 0.23 |
| MBzP | 245 | 99.59 | 0.04 (2.84) | 0.01 | 0.02 | 0.03 | 0.06 | 0.21 |
| MEHP | 245 | 90.2 | 0.01 (3.43) | 0.001 | 0.005 | 0.01 | 0.02 | 0.06 |
| MEOHP | 245 | 97.55 | 0.02 (2.43) | 0.01 | 0.01 | 0.02 | 0.03 | 0.1 |
| MEHHP | 245 | 99.59 | 0.03 (2.5) | 0.01 | 0.02 | 0.03 | 0.06 | 0.18 |
| MECPP | 245 | 64.49 | 0.05 (2.08) | 0.02 | 0.03 | 0.04 | 0.08 | 0.19 |
| Bisphenols (μg/g creatnine) | ||||||||
| BPA | 245 | 80.41 | 0.01 (2.35) | 0.002 | 0.002 | 0.01 | 0.01 | 0.03 |
Abbreviations: LOD, limit of detection; SD, standard deviation. Note: values below the LOD were replaced with LOD divided by the square root of 2.
Figure 1.
Spearman correlations between chemical exposures with >70% detection in the Atlanta African American Maternal-Child study population, 2016-2020 (N=86).
Figure 2.
Change in gestational age (2A) and birthweight z-score (2B) in association with an interquartile range increase in individual chemicals, estimated using linear regression within the Atlanta African American Maternal-Child cohort, 2016-2020 (N=86).
Note: Models are adjusted for maternal education, maternal age, parity, alcohol consumption, and early pregnancy body mass index.
Abbreviations: CI, confidence interval.
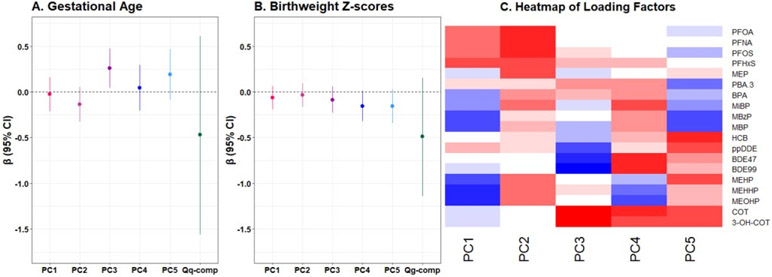
Using quantile g-computation, increasing all exposures in the mixture by one quartile was associated with a modest reduction in both gestational age and birthweight z-scores (mean change per quartile increase= −0.43, 95% CI= −1.56, 0.61; mean change per quartile increase= −0.49, 95% CI= −1.14, 0.15, respectively) (Figure 3; Table S6). MiBP, MEOHP, PFOA, PFOS, and BDE-99 were assigned the largest negative weights in the model which included gestational age as the outcome, while MEHHP, HCB, and COT were assigned the largest negative weights in the model for birthweight z-scores (Figure S2). The overall mixture effect was attenuated when PBDEs and OCPs were removed as exposures from quantile g-computation models (Table S7).
Figure 3.
Adjusted differences in gestational age (3A) and birthweight z-scores (3B) in relation to principal component scores and one quartile increase in the chemical mixture, estimated using quantile g-computation, and (3C) heat map of loading factors from principal component analysis (PCA) of chemical concentrations among pregnant women in the Atlanta African American Maternal-Child cohort, 2016-2020 (N=86).
Note: Models in (3A) and (3B) are adjusted for maternal education, maternal age, parity, alcohol consumption, and early pregnancy body mass index. In (3C), red indicates the range of positive loading factors and blue indicates the range of negative loading factors.
Our PCA identified five meaningful PCs from our exposure data. We characterized each PC by the chemicals that explained the highest variance and were positively associated with each of the PCs (Figure 3). PC1 had high loadings from PFHxS, PFNA, PFOA, and PFOS and we characterized this PC as reflecting high PFAS exposure. PC2 had high loadings from nearly all chemicals. Thus, PC2 reflected general pollution exposure. PC3 had high loadings from COT and 3-OHCOT, and this PC was characterized as reflecting tobacco exposure. PC4 was reflective of exposure to PBDE, nicotine, and certain phthalates, as indicated by the high loadings from BDE-47, BDE-99, cotinine, and MiBP. PC5 reflected exposure to DEHP and insecticides, given the high loadings from MEHHP, MEHP, MEOHP, and p,p’-DDE. We observed that PC3 and PC5 were associated with an increase in gestational age (β= 0.25, 95% CI= 0.04, 0.46; β=0.19, 95% CI= −0.09, 0.47, respectively), while PC2 was associated with a modest reduction. All PCs were associated with a reduction in birthweight z-scores, and the strongest associations were observed with PC4 and PC5 (β= −0.16, 95% CI= −0.32, 0.01; β= −0.16, −0.34, 0.03, respectively) (Figure 3; Table S6).
The univariate exposure-response functions estimated from BKMR showed primarily linear relationships with gestational age and birthweight z-scores (Figure S3 and Figure S4). No individual chemicals were significantly associated with either birth outcome (Figure S5) and we observed a non-significant, inverse association between the overall mixture and birthweight z-scores (Figure S6). BKMR identified both persistent (groupPIP= 0.68) and non-persistent (groupPIP= 0.64) as important exposures groups for birthweight z-scores. When gestational age was the outcome of interest, non-persistent chemicals did not substantially contribute to the overall mixture effect, while persistent chemicals were a moderate contributor (groupPIP= 0.27) (Table S8).
4. Discussion
In the present study, we examined associations between prenatal exposure to multiple classes of environmental chemicals in relation to gestational age and birthweight for gestational age z-scores, a proxy for fetal growth. Using quantile g-computation, a novel method designed for examining exposure mixtures, we observed that increasing exposure to all chemicals was associated with a modest, non-significant, reduction in gestational age and birthweight z-scores. Similarly, using PCA, we observed that the PC reflecting exposure to PFAS, as well as the PC reflecting general pollutant exposure (i.e., high loadings from PFAS, PBDEs, pesticides, insecticides, and phthalates), was associated with slightly reduced fetal growth. In single pollutant models, we observed inconsistent and largely non-significant associations. Our study, conducted among AAs, provides important information regarding the perinatal health effects associated with environmental exposures among a population that is routinely exposed to the highest levels of environmental hazards and experience highest rates of adverse birth outcomes.
Our results are consistent with prior studies that have used single pollutant models to demonstrate prenatal exposure to environmental chemicals is harmful for fetal development. Here, we observed that exposure to BDE-47 and BDE-99 was suggestively associated with reduced fetal growth. This aligns with previous investigations conducted in San Francisco and the Salinas Valley, which observed lower birthweight z-scores and birthweight in relation to increasing BDE-47 and BDE-99.57,58 We similarly found that increasing exposure to p,p’-DDE and HCB was associated with a non-significant reduction in fetal growth, which aligns well with epidemiological evidence from agricultural workers.59 Numerous studies have found that prenatal exposure to phthalates, phenols, OCPs, pyrethroid insecticides, PFAS, and PBDEs is associated with reduced gestational age and increased risk of preterm birth,21,58,60-68 although this was not observed in our study.
A unique aspect of our study was that we quantified exposure levels of multiple classes of environmental chemicals using advanced targeted exposure assessment. This represents an important advancement over prior studies focusing on a single chemical class. Furthermore, we applied three mixtures approaches and incorporated multiple classes of chemical exposures to estimate cumulative effects and identify chemical exposure profiles. The combination of these methods is an important step to increase our understanding of the effects of chemicals on perinatal health and fetal development. Results were similar across methods and suggest that environmental chemicals, particularly persistent organic pollutants, are associated with reduced fetal growth. Our results contribute to a growing body of literature showing that joint exposures to multiple chemicals is associated with an increased risk of adverse health outcomes, and that the effect is greater in magnitude than the effect of a single chemical or single chemical class alone.31,65,69 Prior studies examining chemical mixtures in relation to birth outcomes have produced inconsistent results, which may be reflective of underlying differences in study populations and application of different mixture methods. For example, within the EARTH study, comprised of participants seeking fertility treatment, mixtures of parental (i.e., maternal and paternal) exposures to phthalates and phenols, estimated using BKMR, was associated with an increased risk of preterm birth.70 In the HOME cohort in Cincinnati, exposure to organochlorine pesticides, some phenols, and cadmium as estimated by PCA was associated with reduced birth length, a marker of fetal growth, but not birthweight z-scores, gestational age, or head circumference.31 Exposure to a mixture of PBDEs, PFAS, metals, and OCPs was not strongly associated with fetal growth in a prospective birth cohort in Western Australia.71
We recognize, and attempt to address through this research, that humans are not exposed solely to individual chemicals one at a time. Rather, we interact with environmental chemicals in combination, which may lead to environmental health disparities. There is a growing body of literature showing that communities of color experience a disproportionate burden of toxic chemicals in the environment.72 Compared to whites, Black women are disproportionately targeted by consumer marketing for personal care products that contain mixtures of many of the chemicals included in this analysis, which are known endocrine disrupting toxicants.5 This leads to disparities in exposure, as studies have shown that Black women are more than six times as likely to use hair products that contain endocrine-disrupting chemicals relative to whites.73 Additionally, relative to whites, Black children are more likely to live in neighborhoods that experience increased air pollution due to the ongoing consequences of discriminatory housing practices.33 During pregnancy, non-Hispanic Blacks also have disproportionately higher levels of PBDEs relative to whites and other non-white racial groups.7 Identifying potential causes of exposure disparities in levels of persistent and non-persistent chemicals among Black pregnant people will require research aimed at identifying real-world exposure patterns.
Our results should be interpreted in light of its limitations. Serum lipid data were unavailable in our study population and we are unable to estimate lipid adjusted PBDE concentrations. However, there is debate regarding whether to account for lipid concentrations in the analysis of lipophilic chemicals, and adjustment for serum lipids may induce a spurious association if unknown factors are associated with both adverse pregnancy outcomes and lipid levels.74 Nonetheless, early pregnancy BMI, a common surrogate for adiposity, was retained in our adjusted models, and we conducted sensitivity analyses removing PBDEs from our mixture models. Additionally, we had a relatively small sample size for those who had information on all environmental chemicals available, which may have limited our statistical power. The sample size available for each chemical class was principally driven by the different funding sources and amounts of funding available for assays for various chemical classes. However, we conducted numerous additional analyses, such as removing certain chemical classes from quantile g-computation models, in an effort to increase our sample size (Table S7). We also restricted our statistical analyses to those chemicals that were well-detected, which hinders our ability to make inferences regarding the health effects of chemicals detected at low levels.
Despite these limitations, this study has many important strengths. First, our study population was comprised solely of AAs, a population that is largely excluded from environmental epidemiologic studies yet more likely to be exposed to environmental chemicals. The results from our study may provide important information related to factors contributing to persistent health disparities and the inexplicably high rates of adverse pregnancy outcomes in this population. Additionally, pregnancy outcomes in this study population were based on early pregnancy dating between 8-14 weeks gestation and were ascertained by the abstraction of pregnancy, labor and delivery, and neonatal records by medical personnel, minimizing the chance of outcome misclassification. We also assessed exposure to 43 environmental chemicals and employed mixture methods to assess cumulative effects and create exposure profiles. This represents an important advancement over prior studies that focus on the effects of a single chemical class.
4.1. Conclusions
Among AA pregnant women in Atlanta, Georgia, we observed that increasing exposure to environmental chemicals was associated with suggestive reductions in birthweight for gestational age z-scores. The effects were generally stronger when considering joint exposure to all chemicals, as opposed to single pollutant models assessing the impact of a single chemical one at a time. Our results provide important information regarding the health effects associated with prenatal chemical exposures among a population who experiences high rates of adverse pregnancy outcomes. Future studies should examine associations between chemical mixtures and alternative newborn anthropometric measures (e.g., birth length), as well as focus on identifying upstream predictors of exposure and investigating the molecular mechanisms underlying the chemical toxicities in order to identify opportunities for intervention.
Supplementary Material
Impact statement.
African Americans (AAs) experience higher rates of preterm birth and fetal growth restriction relative to other pregnant populations. Differential in utero exposure to environmental chemicals may partially explain these health disparities, as AAs are disproportionately exposed to environmental hazards. In the present study, we analyzed serum and urine samples for levels of 43 environmental chemicals. We used quantile g-computation, principal component analysis, and BKMR to assess associations between chemical exposure mixtures and adverse birth outcomes. Our findings suggest that prenatal exposure to multiple classes of chemicals is associated with reduced birthweight z-scores, a proxy for fetal growth, in AAs.
Acknowledgements:
We would like to thank all the study participants who participated in this study, and the clinical health care providers and staff at the prenatal recruiting sites for helping with data and sample collection and logistics and sample chemical analyses in the laboratory, especially Nathan Mutic, Estefani Ignacio Gallegos, Nikolay Patrushev, Kristi Maxwell Logue, Castalia Thorne, Shirleta Reid, and Cassandra Hall. We would also like to thank Che-Jung Chang for her assistance with data analysis.
Funding:
This work was supported by the National Institute of Health (NIH) research grants [R01NR014800, R01MD009064, R24ES029490, R01MD009746, R21ES032117], NIH Center Grants [P50ES026071, P30ES019776, UH3OD023318, U2CES026560, U2CES026542, U2COD023375], and Environmental Protection Agency (USEPA) center grant [83615301]. Funding for Stephanie M. Eick was additionally provided from the JPB Environmental Health Fellowship.
Footnotes
Conflicts of interest: None
Data availability statement:
Per Emory University Institutional Review Board approval, the data that support the findings of this study are restricted for transmission to those outside the primary investigative team. Data sharing with investigators outside the team requires IRB approval. Requests may be submitted to the Anne Dunlop, MD, MPH (amlang@emory.edu).
References
- 1.Purisch SE, Gyamfi-Bannerman C. Epidemiology of preterm birth. Semin Perinatol 2017; 41: 387–391. [DOI] [PubMed] [Google Scholar]
- 2.Malhotra A, Allison BJ, Castillo-Melendez M, Jenkin G, Polglase GR, Miller SL. Neonatal Morbidities of Fetal Growth Restriction: Pathophysiology and Impact. Frontiers in Endocrinology 2019; 10.https://www.frontiersin.org/article/10.3389/fendo.2019.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giscombé CL, Lobel M. Explaining disproportionately high rates of adverse birth outcomes among African Americans: the impact of stress, racism, and related factors in pregnancy. Psychol Bull 2005; 131: 662–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braveman P, Dominguez TP, Burke W, Dolan SM, Stevenson DK, Jackson FM et al. Explaining the Black-White Disparity in Preterm Birth: A Consensus Statement From a Multi-Disciplinary Scientific Work Group Convened by the March of Dimes. Frontiers in Reproductive Health 2021; 3.https://www.frontiersin.org/article/10.3389/frph.2021.684207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zota AR, Shamasunder B. The environmental injustice of beauty: framing chemical exposures from beauty products as a health disparities concern. American Journal of Obstetrics and Gynecology 2017; 217: 418.e1–418.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang C-J, Ryan PB, Smarr MM, Kannan K, Panuwet P, Dunlop AL et al. Serum per- and polyfluoroalkyl substance (PFAS) concentrations and predictors of exposure among pregnant African American women in the Atlanta area, Georgia. Environmental Research 2021; 198: 110445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Varshavsky JR, Sen S, Robinson JF, Smith SC, Frankenfield J, Wang Y et al. Racial/ethnic and geographic differences in polybrominated diphenyl ether (PBDE) levels across maternal, placental, and fetal tissues during mid-gestation. Scientific Reports 2020; 10: 12247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Z, Walker GW, Muir DCG, Nagatani-Yoshida K. Toward a Global Understanding of Chemical Pollution: A First Comprehensive Analysis of National and Regional Chemical Inventories. Environ Sci Technol 2020; 54: 2575–2584. [DOI] [PubMed] [Google Scholar]
- 9.Calafat AM, Wong LY, Kuklenyik Z, Reidy JA, Needham LL. Polyfluoroalkyl chemicals in the U.S. population: data from the National Health and Nutrition Examination Survey (NHANES) 2003-2004 and comparisons with NHANES 1999-2000. Environ Health Perspect 2007; 115: 1596–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Silva Manori J, Barr Dana B, Reidy John A, Malek Nicole A, Hodge Carolyn C, Caudill Samuel P et al. Urinary levels of seven phthalate metabolites in the U.S. population from the National Health and Nutrition Examination Survey (NHANES) 1999-2000. Environmental Health Perspectives 2004; 112: 331–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sjödin A, Jones RS, Wong L-Y, Caudill SP, Calafat AM. Polybrominated Diphenyl Ethers and Biphenyl in Serum: Time Trend Study from the National Health and Nutrition Examination Survey for Years 2005/06 through 2013/14. Environ Sci Technol 2019; 53: 6018–6024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sunderland EM, Hu XC, Dassuncao C, Tokranov AK, Wagner CC, Allen JG. A review of the pathways of human exposure to poly- and perfluoroalkyl substances (PFASs) and present understanding of health effects. J Expo Sci Environ Epidemiol 2019; 29: 131–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heudorf U, Mersch-Sundermann V, Angerer J. Phthalates: Toxicology and exposure. International Journal of Hygiene and Environmental Health 2007; 210: 623–634. [DOI] [PubMed] [Google Scholar]
- 14.Geyer HJ, Schramm K-W, Feicht E, Fried K, Henkelmann B, Lenoir D et al. Terminal elimination half-lives of the brominated flame retardants TBBPA, HBCD, and lower brominated PBDEs in humans. 2004. [Google Scholar]
- 15.Jayaraj R, Megha P, Sreedev P. Review Article. Organochlorine pesticides, their toxic effects on living organisms and their fate in the environment. Interdisciplinary Toxicology 2016; 9: 90–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Völkel W, Colnot T, Csanády GA, Filser JG, Dekant W. Metabolism and Kinetics of Bisphenol A in Humans at Low Doses Following Oral Administration. Chem Res Toxicol 2002; 15: 1281–1287. [DOI] [PubMed] [Google Scholar]
- 17.Buckley JP, Kuiper JR, Bennett DH, Barrett ES, Bastain T, Breton CV et al. Exposure to Contemporary and Emerging Chemicals in Commerce among Pregnant Women in the United States: The Environmental influences on Child Health Outcome (ECHO) Program. Environ Sci Technol 2022; 56: 6560–6573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goin DE, Abrahamsson D, Wang M, Park J-S, Sirota M, Morello-Frosch R et al. Investigating geographic differences in environmental chemical exposures in maternal and cord sera using non-targeted screening and silicone wristbands in California. Journal of Exposure Science & Environmental Epidemiology 2022. doi: 10.1038/s41370-022-00426-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morello-Frosch R, Cushing LJ, Jesdale BM, Schwartz JM, Guo W, Guo T et al. Environmental Chemicals in an Urban Population of Pregnant Women and Their Newborns from San Francisco. Environmental Science & Technology 2016; 50: 12464–12472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Woodruff TJ, Zota AR, Schwartz JM. Environmental chemicals in pregnant women in the United States: NHANES 2003-2004. Environ Health Perspect 2011; 119: 878–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Welch BM, Keil AP, Buckley JP, Calafat AM, Christenbury KE, Engel SM et al. Associations Between Prenatal Urinary Biomarkers of Phthalate Exposure and Preterm Birth: A Pooled Study of 16 US Cohorts. JAMA Pediatrics 2022. doi: 10.1001/jamapediatrics.2022.2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bommarito PA, Welch BM, Keil AP, Baker GP, Cantonwine DE, McElrath TF et al. Prenatal exposure to consumer product chemical mixtures and size for gestational age at delivery. Environ Health 2021; 20: 68–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao X, Ni W, Zhu S, Wu Y, Cui Y, Ma J et al. Per- and polyfluoroalkyl substances exposure during pregnancy and adverse pregnancy and birth outcomes: A systematic review and meta-analysis. Environmental Research 2021; 201: 111632. [DOI] [PubMed] [Google Scholar]
- 24.Liu Y, Xie Y, Tian Y, Liu H, He C, An S et al. Associations Between Polybrominated Diphenyl Ethers Concentrations in Human Placenta and Small for Gestational Age in Southwest China. Frontiers in Public Health 2022; 10. doi: 10.3389/fpubh.2022.812268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Panagopoulos Abrahamsson D, Wang A, Jiang T, Wang M, Siddharth A, Morello-Frosch R et al. A Comprehensive Non-targeted Analysis Study of the Prenatal Exposome. Environ Sci Technol 2021; 55: 10542–10557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lampa E, Eguchi A, Todaka E, Mori C. Fetal exposure markers of dioxins and dioxin-like PCBs. Environmental Science and Pollution Research 2018; 25: 11940–11947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ashrap P, Watkins DJ, Mukherjee B, Boss J, Richards MJ, Rosario Z et al. Maternal blood metal and metalloid concentrations in association with birth outcomes in Northern Puerto Rico. Environ Int 2020; 138: 105606–105606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cathey AL, Eaton JL, Ashrap P, Watkins DJ, Rosario ZY, Vélez Vega C et al. Individual and joint effects of phthalate metabolites on biomarkers of oxidative stress among pregnant women in Puerto Rico. Environment International 2021; 154: 106565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boss J, Zhai J, Aung MT, Ferguson KK, Johns LE, McElrath TF et al. Associations between mixtures of urinary phthalate metabolites with gestational age at delivery: a time to event analysis using summative phthalate risk scores. Environmental Health 2018; 17: 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu JMY, Arbuckle TE, Janssen P, Lanphear BP, Zhuang LH, Braun JM et al. Prenatal exposure to endocrine disrupting chemical mixtures and infant birth weight: A Bayesian analysis using kernel machine regression. Environmental Research 2021; 195: 110749. [DOI] [PubMed] [Google Scholar]
- 31.Kalloo G, Wellenius GA, McCandless L, Calafat AM, Sjodin A, Romano ME et al. Exposures to chemical mixtures during pregnancy and neonatal outcomes: The HOME study. Environment International 2020; 134: 105219. [DOI] [PubMed] [Google Scholar]
- 32.Cabrera-Rodríguez R, Luzardo OP, Almeida-González M, Boada LD, Zumbado M, Acosta-Dacal A et al. Association between prenatal exposure to multiple persistent organic pollutants (POPs) and growth indicators in newborns. Environmental Research 2019; 171: 285–292. [DOI] [PubMed] [Google Scholar]
- 33.Hwa Jung K, Pitkowsky Z, Argenio K, Quinn JW, Bruzzese J-M, Miller RL et al. The effects of the historical practice of residential redlining in the United States on recent temporal trends of air pollution near New York City schools. Environment International 2022; 169: 107551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ray K In the Name of Racial Justice: Why Bioethics Should Care about Environmental Toxins. Hastings Center Report 2021; 51: 23–26. [DOI] [PubMed] [Google Scholar]
- 35.Corwin EJ, Hogue CJ, Pearce B, Hill CC, Read TD, Mulle J et al. Protocol for the Emory University African American Vaginal, Oral, and Gut Microbiome in Pregnancy Cohort Study. BMC Pregnancy and Childbirth 2017; 17: 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brennan PA, Dunlop AL, Smith AK, Kramer M, Mulle J, Corwin EJ. Protocol for the Emory University African American maternal stress and infant gut microbiome cohort study. BMC Pediatrics 2019; 19: 246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hornung RW, Reed LD. Estimation of Average Concentration in the Presence of Nondetectable Values. Applied Occupational and Environmental Hygiene 1990; 5: 46–51. [Google Scholar]
- 38.Barr Dana B, Wilder Lynn C, Caudill Samuel P, Gonzalez Amanda J, Needham Lance L, Pirkle James L Urinary Creatinine Concentrations in the U.S. Population: Implications for Urinary Biologic Monitoring Measurements. Environmental Health Perspectives 2005; 113: 192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Balshaw DM, Collman GW, Gray KA, Thompson CL. The Children’s Health Exposure Analysis Resource: enabling research into the environmental influences on children’s health outcomes. Curr Opin Pediatr 2017; 29: 385–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Honda M, Robinson M, Kannan K. A rapid method for the analysis of perfluorinated alkyl substances in serum by hybrid solid-phase extraction. Environ Chem 2018; 15: 92–99. [Google Scholar]
- 41.Darrow LA, Jacobson MH, Preston EV, Lee GE, Panuwet P, Hunter RE Jr et al. Predictors of serum polybrominated diphenyl ether (PBDE) concentrations among children aged 1–5 years. Environmental science & technology 2017; 51: 645–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jacobson MH, Barr DB, Marcus M, Muir AB, Lyles RH, Howards PP et al. Serum polybrominated diphenyl ether concentrations and thyroid function in young children. Environmental research 2016; 149: 222–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mutic AD, Barr DB, Hertzberg VS, Brennan PA, Dunlop AL, McCauley LA. Polybrominated Diphenyl Ether Serum Concentrations and Depressive Symptomatology in Pregnant African American Women. International Journal of Environmental Research and Public Health 2021; 18. doi: 10.3390/ijerph18073614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marder ME, Panuwet P, Hunter RE, Ryan PB, Marcus M, Barr DB. Quantification of Polybrominated and Polychlorinated Biphenyls in Human Matrices by Isotope-Dilution Gas Chromatography–Tandem Mass Spectrometry. Journal of Analytical Toxicology 2016; 40: 511–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Olsson AO, Baker SE, Nguyen JV, Romanoff LC, Udunka SO, Walker RD et al. A Liquid Chromatography–Tandem Mass Spectrometry Multiresidue Method for Quantification of Specific Metabolites of Organophosphorus Pesticides, Synthetic Pyrethroids, Selected Herbicides, and DEET in Human Urine. Anal Chem 2004; 76: 2453–2461. [DOI] [PubMed] [Google Scholar]
- 46.Zhang X, Barr DB, Dunlop AL, Panuwet P, Sarnat JA, Lee GE et al. Assessment of metabolic perturbations associated with exposure to phthalates among pregnant African American women. Science of The Total Environment 2021; : 151689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou X, Kramer JP, Calafat AM, Ye X. Automated on-line column-switching high performance liquid chromatography isotope dilution tandem mass spectrometry method for the quantification of bisphenol A, bisphenol F, bisphenol S, and 11 other phenols in urine. Journal of Chromatography B 2014; 944: 152–156. [DOI] [PubMed] [Google Scholar]
- 48.Fisher Toutenburg H., A. R, and Yates F: Statistical Tables for Biological, Agricultural and Medical Research. 6th Ed. Oliver & Boyd, Edinburgh and London: 1963. X, 146 P. Preis 42 s net. Biometrische Zeitschrift 1971; 13: 285–285. [Google Scholar]
- 49.Knuth DE. The Art of Computer Programming, Volume 2 (3rd Ed.): Seminumerical Algorithms. Addison-Wesley Longman Publishing Co., Inc.: USA, 1997. [Google Scholar]
- 50.Yakimavets V, Qiu T, Panuwet P, D’Souza PE, Brennan PA, Dunlop AL et al. Simultaneous quantification of urinary tobacco and marijuana metabolites using solid-supported liquid-liquid extraction coupled with liquid chromatography tandem mass spectrometry. Journal of Chromatography B 2022; 1208: 123378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Committee Opinion No 700: Methods for Estimating the Due Date. Obstetrics & Gynecology 2017; 129. https://journals.lww.com/greenjournal/Fulltext/2017/05000/Committee_Opinion_No_700__Methods_for_Estimating.50.aspx. [DOI] [PubMed] [Google Scholar]
- 52.Aris IM, Kleinman KP, Belfort MB, Kaimal A, Oken E. A 2017 US Reference for Singleton Birth Weight Percentiles Using Obstetric Estimates of Gestation. Pediatrics 2019; 144: e20190076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Keil AP, Buckley JP, O’Brien KM, Ferguson KK, Zhao S, White AJ. A Quantile-Based g-Computation Approach to Addressing the Effects of Exposure Mixtures. Environ Health Perspect 2020; 128: 47004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bobb JF, Claus Henn B, Valeri L, Coull BA. Statistical software for analyzing the health effects of multiple concurrent exposures via Bayesian kernel machine regression. Environmental Health 2018; 17: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bobb JF, Valeri L, Claus Henn B, Christiani DC, Wright RO, Mazumdar M et al. Bayesian kernel machine regression for estimating the health effects of multi-pollutant mixtures. Biostatistics 2015; 16: 493–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Darbre PD. Chapter 15 - Endocrine Disruption and Disorders of Energy Metabolism. In: Darbre PD (ed). Endocrine Disruption and Human Health. Academic Press: Boston, 2015, pp 273–285. [Google Scholar]
- 57.Eick SM, Hom Thepaksorn EK, Izano MA, Cushing LJ, Wang Y, Smith SC et al. Associations between prenatal maternal exposure to per- and polyfluoroalkyl substances (PFAS) and polybrominated diphenyl ethers (PBDEs) and birth outcomes among pregnant women in San Francisco. Environ Health 2020; 19: 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Harley KG, Chevrier J, Schall RA, Sjödin A, Bradman A, Eskenazi B. Association of Prenatal Exposure to Polybrominated Diphenyl Ethers and Infant Birth Weight. American Journal of Epidemiology 2011; 174: 885–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Eskenazi Brenda, Harley Kim, Bradman Asa, Weltzien Erin, Jewell Nicholas P, Barr Dana B et al. Association of in Utero Organophosphate Pesticide Exposure and Fetal Growth and Length of Gestation in an Agricultural Population. Environmental Health Perspectives 2004; 112: 1116–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhong Q, Liu H, Fu H, Niu Q, Wu H, Huang F. Prenatal exposure to phthalates with preterm birth and gestational age: A systematic review and meta-analysis. Chemosphere 2021; 282: 130991. [DOI] [PubMed] [Google Scholar]
- 61.Ferguson KK, McElrath TF, Meeker JD. Environmental Phthalate Exposure and Preterm Birth. JAMA Pediatrics 2014; 168: 61–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ferguson KK, Rosen EM, Barrett ES, Nguyen RHN, Bush N, McElrath TF et al. Joint impact of phthalate exposure and stressful life events in pregnancy on preterm birth. Environment International 2019; 133: 105254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Santos S, Sol CM, van Zwol – Janssens C, Philips EM, Asimakopoulos AG, Martinez-Moral M-P et al. Maternal phthalate urine concentrations, fetal growth and adverse birth outcomes. A population-based prospective cohort study. Environment International 2021; 151: 106443. [DOI] [PubMed] [Google Scholar]
- 64.Namat A, Xia W, Xiong C, Xu S, Wu C, Wang A et al. Association of BPA exposure during pregnancy with risk of preterm birth and changes in gestational age: A meta-analysis and systematic review. Ecotoxicology and Environmental Safety 2021; 220: 112400. [DOI] [PubMed] [Google Scholar]
- 65.Deji Z, Liu P, Wang X, Zhang X, Luo Y, Huang Z. Association between maternal exposure to perfluoroalkyl and polyfluoroalkyl substances and risks of adverse pregnancy outcomes: A systematic review and meta-analysis. Science of The Total Environment 2021; 783: 146984. [DOI] [PubMed] [Google Scholar]
- 66.Peltier MR, Fassett MJ, Arita Y, Chiu VY, Shi JM, Takhar HS et al. Women with high plasma levels of PBDE-47 are at increased risk of preterm birth. Journal of Perinatal Medicine 2021; 49: 439–447. [DOI] [PubMed] [Google Scholar]
- 67.Bell GA, Perkins N, Buck Louis GM, Kannan K, Bell EM, Gao C et al. Exposure to Persistent Organic Pollutants and Birth Characteristics: The Upstate KIDS Study. Epidemiology 2019; 30.https://journals.lww.com/epidem/Fulltext/2019/11001/Exposure_to_Persistent_Organic_Pollutants_and.13.aspx. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Naksen W, Prapamontol T, Mangklabruks A, Chantara S, Thavornyutikarn P, Srinual N et al. Associations of maternal organophosphate pesticide exposure and PON1 activity with birth outcomes in SAWASDEE birth cohort, Thailand. Environmental Research 2015; 142: 288–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ouidir M, Buck Louis GM, Kanner J, Grantz KL, Zhang C, Sundaram R et al. Association of Maternal Exposure to Persistent Organic Pollutants in Early Pregnancy With Fetal Growth. JAMA Pediatrics 2020; 174: 149–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang Y, Mustieles V, Williams PL, Wylie BJ, Souter I, Calafat AM et al. Parental preconception exposure to phenol and phthalate mixtures and the risk of preterm birth. Environment International 2021; 151: 106440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lazarevic N, Barnett AG, Sly PD, Callan AC, Stasinska A, Heyworth JS et al. Prenatal exposure to mixtures of persistent environmental chemicals and fetal growth outcomes in Western Australia. International Journal of Hygiene and Environmental Health 2022; 240: 113899. [DOI] [PubMed] [Google Scholar]
- 72.Bell Michelle L, Ebisu Keita. Environmental Inequality in Exposures to Airborne Particulate Matter Components in the United States. Environmental Health Perspectives 2012; 120: 1699–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.James-Todd T, Senie R, Terry MB. Racial/Ethnic Differences in Hormonally-Active Hair Product Use: A Plausible Risk Factor for Health Disparities. Journal of Immigrant and Minority Health 2012; 14: 506–511. [DOI] [PubMed] [Google Scholar]
- 74.Schisterman Enrique F, Whitcomb Brian W, Buck Louis Germaine M, Louis Thomas A Lipid Adjustment in the Analysis of Environmental Contaminants and Human Health Risks. Environmental Health Perspectives 2005; 113: 853–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Per Emory University Institutional Review Board approval, the data that support the findings of this study are restricted for transmission to those outside the primary investigative team. Data sharing with investigators outside the team requires IRB approval. Requests may be submitted to the Anne Dunlop, MD, MPH (amlang@emory.edu).