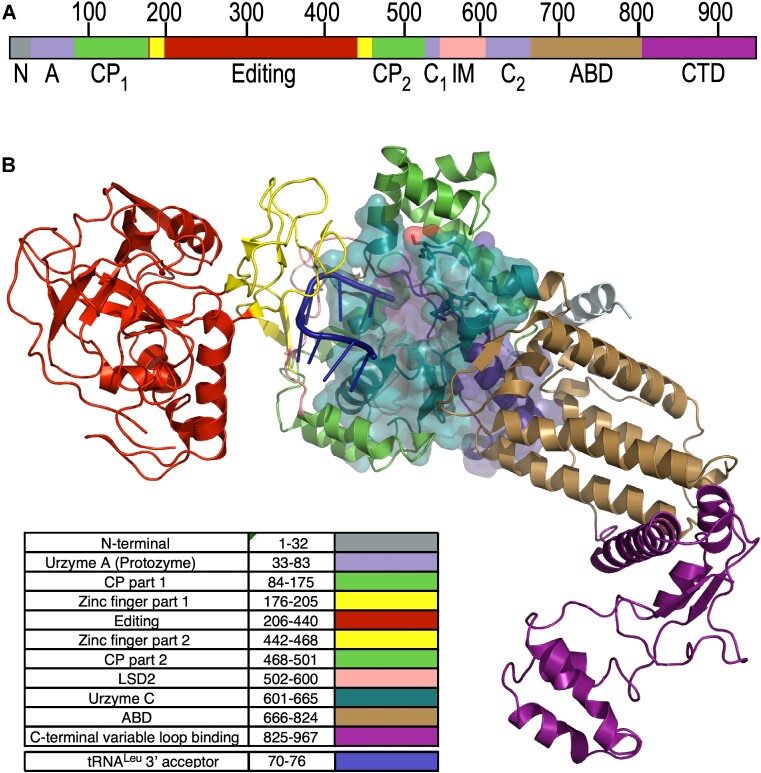
Figure 1.
Relation of LeuAC urzyme to full-length P. horikoshii LeuRS. Colors are consistent between A and B. (A) Linear schematic of LeuRS domains. N,N-terminal helical domain; A, Protozyme (ATP-binding site); CP, connecting peptide, a nested insertion containing the editing domain; Editing, Editing domain; C1, second fragment of the urzyme (amino acid and tRNA binding). LSD2, Leucine specific domain 2 (11), an insertion module before second crossover; C2, C-terminal fragment of the urzyme (pyrophosphate binding); ABD, anticodon-binding domain; CTD, C-terminal domain that binds to the tRNALeu variable loop. (B) Three-dimensional cartoon based on PDB ID 1WC2 showing the LeuAC urzyme (surface embedded into the full-length enzyme and the arrangement of modules acquired during the specialization of LeuRS from other Class I aaRS. A76 of the tRNALeu acceptor stem inserts into the active sites of both LeuRS and LeuAC, bisecting the protozyme (blue) and urzyme part C (C2), the second half of the urzyme. Insertions into a loop connecting the protozyme and C2 are all nested, one within the next, forming the complete CP. In contrast, C-terminal additions are serial.