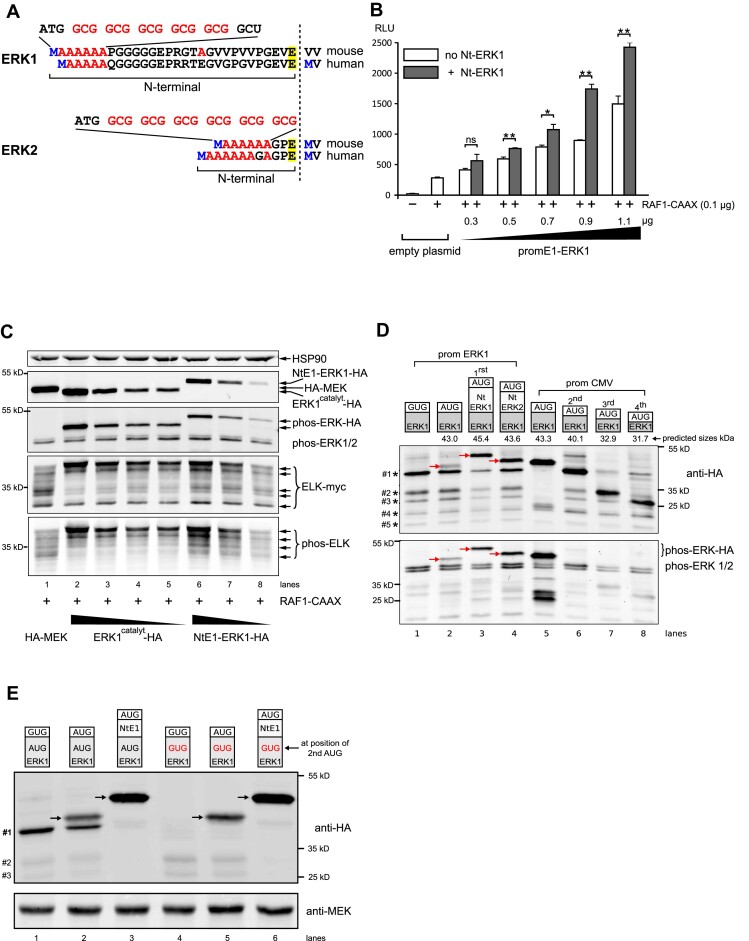
Figure 2.
Effect of ERK NTARs on ERK signaling, activity and expression. (A) Nucleotide and amino acid composition of mouse and human ERK1 and ERK2 Nt domains upstream of the glutamic acid residue common to both ERKs (highlighted in yellow). Downstream of the initiating methionine (in blue), alanine stretches occur and their corresponding GCG codons are highlighted in red (full nucleotide sequence in Supplementary Figure S10). (B) Measurement of ERK activity using the GAL4-ELK/Luc system (RLU) in HeLa cells transfected with an empty plasmid or increasing quantities of ERK1 under the control of its own proximal promoter, in the absence (white bars) or presence of its Nt moiety (gray bars). Except for the first empty plasmid condition, cells were all co-transfected with 100 ng of constitutive active RAF1-CAAX construct (n = 3; *P < 0.05 or **P < 0.01 bilateral Welch's t-test, representative of three experiments). (C) Western blot analysis from extracts of HeLa cells transfected with active RAF1-CAAX and myc-tagged Gal4-ELK (ELK-myc), together with HA-MEK plasmid (lane 1) or decreasing amounts of HA-tagged ERK1, either with its Nt (NtE1-ERK1-HA, lanes 6–8) or without (ERK1catalyt-HA, the catalytic moiety of mouse ERK1 lacking the first 26 amino acids, lanes 2–5). The top panel shows expression of HSP90 as a loading control, the second panel shows expression of ERK1-HA and HA-MEK, the third panel reveals phosphorylated ERKs (ectopic and endogenous), and the fourth and fifth panels show the profile of total myc-tagged GAL4-ELK and the multi-phosphorylated GAL4-ELK profile. (D) Western blot analysis from extracts of HEK293 cells transfected with various HA-tagged ERK constructs depicted with their predicted molecular weights above the blots. One day post-transfection, cells were treated overnight with 100 nM bortezomib to decrease proteasome-mediated protein degradation, then cells were stimulated for 15 min with 0.1 M orthovanadate prior to lysis to increase detection of phosphorylated ERK. The first four constructs were expressed under the erk1 promoter (lanes 1–4). ERK1 was expressed from either a mutated AUG (GUG, lane 1) or a normal AUG initiation codon (lanes 2–4). Nt domains of ERK1 or ERK2 were fused to catalytic ERK1 (lanes 3 and 4). Truncated ERK1 forms were expressed under the CMV promoter (lanes 5–8). Of note, lanes 5–8 were transfected with 18-fold less plasmid than lanes 1–4 (CMV versus ERK1 promoter). Lane 5 shows catalytic ERK1, whereas lanes 6–8 show polypeptides starting at the second, third or fourth in-frame AUG codons. Red arrows indicate the full-length ERK1-HA proteins. In lanes 1–4, smaller polypeptides expressed under the erk1 promoter are indicted by decreasing sizes (#1 to #5) on the left of the upper blot. Detection of HA-tagged proteins was performed using an anti-HA antibody (upper panel), whereas phosphorylated ERK (ectopic and endogenous) was assessed using a specific phospho-ERK antibody (lower panel). (E) Western blot analysis of HeLa cells transfected and processed as in (D). The first three lanes show expression of ERK1-HA whose catalytic domain is WT while the last three lanes show expression of ERK1-HA whose catalytic domain has the second in-frame AUG mutated to GUG. In lanes 1 and 4, ERK1 lacks its first AUG, while in lanes 3 and 6 ERK1 contains the Nt domain. Arrows indicate the functional ERK1-HA proteins. The lower molecular weight polypeptides are indicated on the left (#1 to #3).