Abstract
Background:
Insulin resistance (IR) is a pathological condition in which cells fail to respond normally to insulin. IR has been associated with multiple conditions, including chronic pain. Fibromyalgia (FM) is one of the common generalized chronic painful conditions with an incidence rate affecting 3% to 6% of the population. Substantial interest and investigation into FM continue to generate many hypotheses.
The relationship between IR and FM has not been explored. IR is known to cause abnormalities in the cerebral microvasculature, leading to focal hypoperfusion. IR also has been shown to cause cognitive impairment in FM patients, as in parkinsonism. As demonstrated by advanced imaging methods, similar brain perfusion abnormalities occur in the brain of patients with FM as with IR.
Objectives:
To determine the potential association between FM and IR.
Setting:
Subspecialty pain medicine clinics.
Study Design:
Observational cross-sectional study.
Methods:
Laboratory data was extracted through a retrospective review of medical records from patients who had met the American College of Rheumatology (ACR) criteria for FM. The Hemoglobin A1c (HbA1c) values from 33 patients with FM were compared with the means of the glycated HbA1c levels of 2 control populations. In addition, established indices of IR [Quantitative Insulin Sensitivity Check Index (QUICKI) and the Homeostatic Model Assessment of Insulin Resistance (HOMA-IR)] were calculated in a subgroup of patients in whom the analytes necessary for these calculations were available. To assess for confounding factors, the associations between HbA1c, QUICKI, HOMA-IR, fasting insulin levels, and glucose, after controlling for age, were explored by multiple analyses of variance with relation to gender and ethnicity.
Results:
We found an association between IR and FM that was independent of age, gender, and ethnicity. We found that patients with FM belong to a distinct population that can be segregated from the control groups by their HbA1c levels, a surrogate marker of IR. This was demonstrated by analyzing the data after introducing an age correction into a linear regression model. This strategy showed significant differences between patients with FM and control subjects (P < 0.0001 and P = 0.0002, for 2 separate control populations, respectively). A subgroup analysis using the QUICKI and HOMA-IR showed that all patients with FM in this subgroup (100%) exhibited laboratory abnormalities pointing to IR.
Limitations:
Small observational cross-sectional study. There are also intrinsic limitations that are attributed to cross-sectional studies.
Conclusion:
The association demonstrated in this study warrant further investigation, including the pursuit of randomized, double-blind clinical trials to determine the effect of improving insulin sensitivity in FM related pain scores. Such studies could unveil a potential pathogenetic relationship between FM, central pain, and IR. Based on these initial findings, we present the hypothesis that IR may underlie pathological mechanisms leading to central pain. If confirmed, this may lead to a paradigm shift in the management of central pain.
Keywords: Fibromyalgia, insulin resistance, chronic widespread pain, hemoglobin A1c
Insulin resistance (IR) is defined clinically as the inability of a known quantity of exogenous or endogenous insulin to increase glucose uptake utilization in an individual as much as it does in a normal population (1,2). Joslin (3) in 1916 recognized hyperglycemic situations after infectious diseases, painful conditions such as gallstones, and trauma. Eight years later, in 1924, Rabinowitch observed that diabetic patients needed more insulin during infection (4). During the same period in 1920, Pemberton and Foster described impaired glucose regulation in soldiers with arthritis (5). Root (6) in 1929 coined the term “insulin resistance” (IR) to describe the presence of an inadequately high need for insulin in different diseases. Over the last century, IR was found in conditions such as diabetes mellitus, obesity, infection, sepsis, arthritis of various types, systemic lupus erythematosus, ankylosing spondylitis, trauma, postoperative pain, migraine, schizophrenia, major depression, and mental stress, to name the most important (2). In the 1950s, combined glucose and insulin tests demonstrated IR in chronic inflammatory diseases, such as rheumatoid arthritis (6–8).
Evolving work in rheumatology and chronic pain has recognized IR in many pain conditions, including migraine, neuropathic pain, and multiple other conditions leading to chronic pain (9–13). Also, IR is associated with dementia in patients with Parkinson’s disease and cognitive impairment with fibromyalgia (FM) (14,15).
FM is a painful syndrome characterized by central pain, manifested as chronic widespread musculoskeletal pain, stiffness, and multiple tender points as defined by the International Association for the Study of Pain (IASP) (16). FM involves disordered afferent processing with central sensitization and a heightened pain response (17–22). Thus, FM is defined as a “central sensitization syndrome”, characterized by central sensitization or central “brain” pain (17,18). While widespread pain is the defining characteristic of FM, the disorder is frequently associated with depression, fatigue, sleep disturbances, and disability, which essentially contribute to the usual physical inactivity (23,24). Patients with FM often complain of memory, concentration and attention deficits, which in some patients are severe enough to be referred to as “fibro-fog” or “brain fog” (17–26). In fact, neuropsychological studies in FM have shown deficits affecting various cognitive domains (17,18,20,21,25,26).
The lack of understanding of the etiology and pathogenesis of FM leads to difficult management of these patients. In addition, patients with FM commonly have an elevated prevalence of overweight or obesity (27), diabetes mellitus (28), and metabolic syndrome (29). Previous studies have shown that IR may increase the risk of cognitive impairment not only in diabetes mellitus (30,31), but also in Alzheimer’s disease (32,33), Parkinson’s disease (14), migraine (9), obesity (27,34), women (35), and FM (15). In a study of IR as a possible risk factor for cognitive impairment in FM patients, Fava et al (15) showed that IR was present in 79% of the patients of whom 23% also had impaired glucose tolerance, 4% newly diagnosed with diabetes mellitus, and 52% IR only. Experimental evidence showed a correlative relationship between chronic pain and IR in Zucker fatty rats; this model showed a bidirectional relationship between pain and IR (11). The investigators postulated that a decreased expression of insulin receptors in skeletal muscle innervated by the injured nerve is one of the underlying mechanisms (11). García et al (13) also showed fructose-induced IR as a model of neuropathic pain in rats. They demonstrated that IR induced by fructose reproduced several aspects of neuropathic pain, suggesting that nociceptive hypersensitivity in this model is due to the modulation of several ionic channels as the primary afferent neurons. Advanced imaging methods have shown that IR leads to dysfunctions in the brain microcirculation, resulting in cerebral hypoperfusion (34). It is interesting to note that focal deficits in brain perfusion have also been observed in advanced imaging of patients with FM (35). Pappolla et al (36) recently published a preliminary report suggested IR as a possible cause of FM. They showed that a subgroup of patients who had undergone treatment with metformin experienced improvements in their widespread myofascial pain (see Disclosure).
FM is among the most frequent generalized chronic pain disorders (23,24,37,38). The literature shows that approximately 10% to 12% of the general population has widespread chronic pain (37,38), whereas FM affects 3% to 6% of the population (23,24,37,38). FM may also be associated with many other disorders, including spinal pathology, inflammatory bowel disease, diabetes, obesity, and metabolic syndrome (27,28). FM is often exacerbated by multiple stressors and psychological components, which may be part of the disorder itself (39–42). However, there is no disease-modifying treatment for FM. Thus far, numerous modalities of treatments are available for symptomatic improvement, but pain relief is not achieved in a significant proportion of patients despite pharmacological and non-pharmacological approaches available (43–47). Therefore, the economic burden of other painful musculoskeletal conditions, excluding neck and back pain, are enormous and have been shown to be the second most common condition with expenditures of $129.8 billion in 2016, an increase of 35% from 2013 of $95.9 billion (48,49). These patients have a higher prevalence of back and neck pain, in addition to FM (39), further increasing the economic burden of FM (50–52).
FM is hypothesized to be a central sensitivity pain disorder primarily characterized by pathological processing of nociceptive stimuli (53). Numerous hypotheses have been put forth to explain the extensive and diverse array of symptoms, including inherited abnormalities (54), dysfunction of neurotransmitters pathways such as substance P (54,55), immune dysregulation (55–57), and several other theories (55); however, the pathophysiology of FM remains an enigma. The literature also shows that IR causes focal cerebral hypoperfusion (34), and focal cerebral hypoperfusion is present in patients with FM (35). This mechanism leads to our hypothesis that IR may be associated with the pathophysiologic basis of FM. Links to IR were previously demonstrated in patients having FM with cognitive dysfunction and in patients with chronic pain of various etiologies, including migraine (36). The previously shown relationship between IR and FM and the improvement of myofascial pain with metformin encouraged us to undertake this retrospective study to strengthen our findings by enlarging the sample and incorporating additional markers of IR in a subgroup of patients with FM (36).
Methods
Sample Description
All patients with FM, were patients in a subspecialty pain medicine clinic located in Houston, Texas. A retrospective review of medical records was performed independently by 2 authors (MAP and FA). We selected patients who had widespread myofascial pain and had met the 1990, as well as the 2010/2011 American College of Rheumatology (ACR) criteria for FM diagnosis (i.e., we retained tender points in the evaluation), for further analysis, after excluding the following patients:
Patients with comorbid disorders that could cause chronic multifocal pain through other mechanisms such as rheumatoid arthritis, other inflammatory rheumatological arthropathies, lupus, and autoimmune diseases. The latest 2016 ACR criteria for FM diagnosis allows for the inclusion of such comorbid disorders in FM (i.e., “rheumatoid arthritis with FM,” etc.) (58).
Patients on medications that could worsen IR, such as glucocorticoids, thiazide diuretics, beta-blockers, and antipsychotics.
Patients with Hemoglobin A1c (HbA1c) determinations performed only by point of care (POC) methodology.
Several studies have reported an association between small fiber neuropathy and FM (59,60). For this reason, many patients with FM in our clinics routinely undergo a laboratory workup for peripheral neuropathy, which includes HbA1c values and, less frequently, fasting insulin levels.
The HbA1c values from 33 patients with FM (17 Hispanic; 10 White; 6 African American) were compared with the means of the HbA1c levels of 2 populations used as controls. One population consisted of nondiabetic individuals with normal glucose tolerance (obtained from the Framingham Offspring Study (FOS) NGT) for ages shown in Fig. 1. The second population consisted of the National Health and Nutrition Examination Survey (NHANES) nondiabetic dataset obtained from the Centers for Disease Control (CDC). Descriptions of these control populations have been published (61).
Fig. 1.
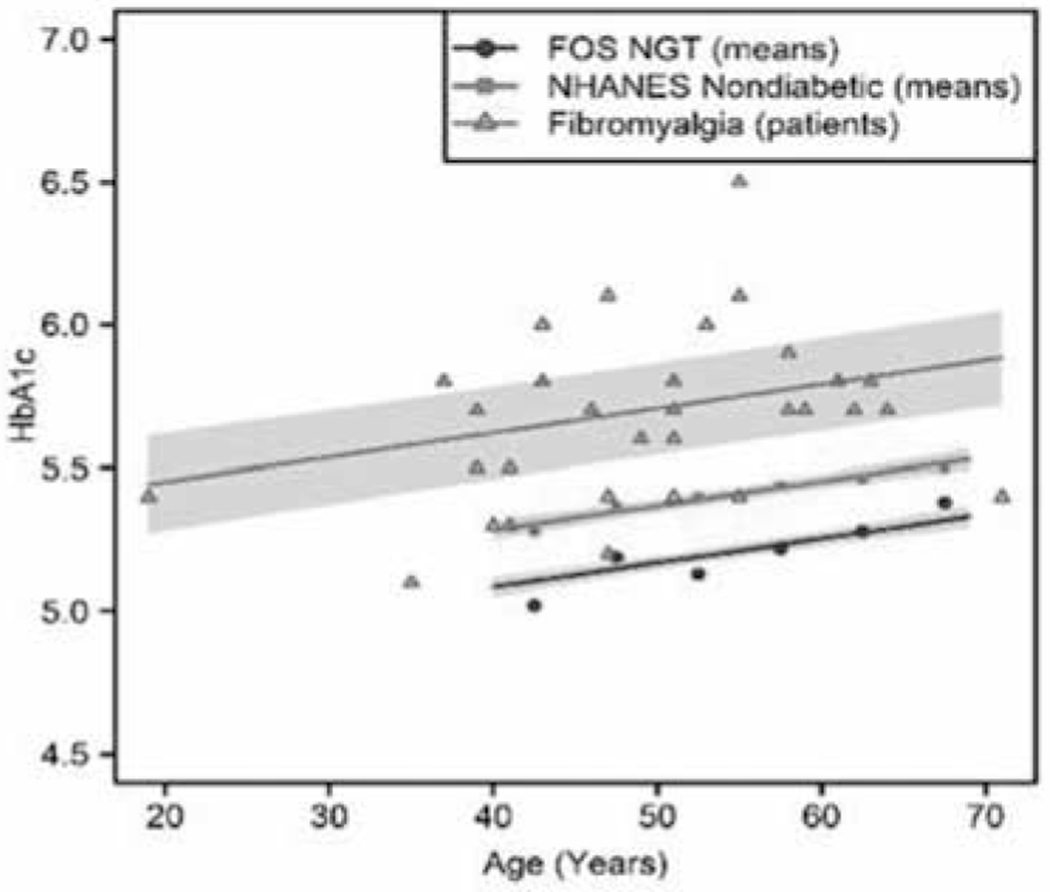
HbA1c values in 33 patients with FM (17 Hispanic; 10 White; 6 African American) were compared with the means of a nondiabetic population with normal glucose tolerance (obtained from the Framingham Offspring Study) and a nondiabetic group from the NHANES study. Regression lines are shown with shaded 95% confidence regions. FOS NGT and NHANES nondiabetic HbA1c include scatterplots of published mean values for each age region, and while FM includes a scatterplot of measures from individual patients (several overlap in values). The regression estimates that FM HbA1c averages .54 +/− .08 (mean +/−SE) units higher than FOS NGT (P < 0.0001), and .34 +/− .08 units higher than NHANES nondiabetic patients, P = 0.0001.
All laboratory investigations were performed by independent Clinical Laboratory Improvement Act (CLIA) accredited laboratories (62) and included routine chemistry panels, HbA1c (n = 33), and fasting insulin levels (n = 13). As controls for the HbA1c results, we used the values obtained from individuals enrolled in the FOS (63). Although available in our clinics, we also excluded patients studied by POC methodology. The reasons for this are as follows: 1-The CLIA accreditation requires the inclusion of internal and external control samples for testing, normal and abnormal, and provides a more reliable range for interpretation of abnormal results. 2-Inclusion of internal control samples from healthy patients in the same community is required by CLIA and assayed in the same manner as the patient specimens; therefore, this obviates the inclusion of internal control samples obtained from our clinics, a challenging endeavor in a subspecialty clinical setting. 3-Normal and abnormal external quality control samples are also required, which allow for meaningful interlaboratory comparisons of our results.
In 13 patients with FM, we had sufficient information to calculate the quantitative insulin sensitivity check index (QUICKI) (64) and the homeostatic model assessment for IR (HOMA-IR) (65).
Statistical Analysis
To formally compare the HbA1c measures in patients with FM to the FOS NGT and NHANES nondiabetic data published in Table 1 of Pani et al (61) (while realistically characterizing the variation contributed from these patients), we generated simulated sets of HbA1c data to emulate the FOS NGT and NHANES source populations in Table 1. From Pani et al.’s data (61), the values for each age group for N, mean, and standard error over the age range from 40 to 69 years, were used as the basis of estimates of the standard deviation (we estimated the corresponding standard deviation as the product of the standard error and square root of N for each age group), separately for FOS NGT and NHANES nondiabetic groups. Per these means and standard deviations, we produced a random normally distributed data set to simulate the source population of the data. We verified that the means and standard errors of the simulated data agreed well with the values in the tables over each age range; estimates of the means were within 0.1%, and standard errors were within 4%. We paired the simulated FOS NGT and NHANES HbA1c source control data with HbA1c measures in patients with FM, and then we used linear regression to model the association between HbA1c with age and group (simulated FOS HGT patients (n = 1350), simulated NHANES nondiabetic patients (n = 1592), and patients with FM (n = 33). Tukey (66) estimated adjusted differences among the groups. A model was also considered, which included an interaction between age and group; however, this yielded a worse model due to increased Akaike Information Criterion (67) as well as a lack of significance of the interaction term.
Table 1.
Differences in HbA1c among groups, per Tukey-adjusted differences from regression model.
| Estimate | SE | P-value | |
|---|---|---|---|
| NHANES Nondiabetic - FOS NGT | 0.20 | 0.02 | < 0.0001 |
| FM - FOS NGT | 0.54 | 0.08 | < 0.0001 |
| FM - NHANES Nondiabetic | 0.34 | 0.08 | 0.0001 |
To assess the associations between HbA1c, QUICKI, HOMA-IR, fasting insulin levels, glucose, with gender and ethnicity, controlling for age, each factor was modeled by multiple analysis of variance with relation to gender, ethnicity, and age. We used the R statistical software for the analyses (R Core Team, 2020, version 3.6.3). Catseye plots utilized the catseyes package (68,69). All tests assumed a 95% level of confidence, with α = .05.
Ethics Statement
This study consisted of a retrospective review of anonymized medical records and was determined to be exempt from review according to US regulation 45 CFR §46.104 Category #4. This determination was made by an independent accredited Investigational Review Board (IntegReview, Austin, TX).
Results
Most patients afflicted with FM were segregated from 2 control groups by its HbA1c values, a surrogate marker of IR. Most importantly, and unique to our research, we analyzed the data for the HbA1c after having introduced an age covariate adjustment into a linear regression model; this approach revealed highly significant differences between the patients and the controls (P < 0.0001 and P = 0.0001, for 2 separate control populations, respectively). The reasons for this approach to the analysis are explained in the Discussion section of this paper.
The regression relating HbA1c to group (FM HbA1c, FOS NGT, NHANES nondiabetic), summarized in Table 1 and Fig. 1, showed that patients with FM average .54 units of A1c higher than FOS NGT, P < .0001, and that patients with FM average .34 units higher than NHANES nondiabetic, P = .0001.
Analyses of variance relating HbA1c, QUICKI, HOMA-IR, fasting insulin levels, and fasting glucose to gender and ethnicity found no significant evidence of association, as illustrated in Fig. 2.
Fig. 2.
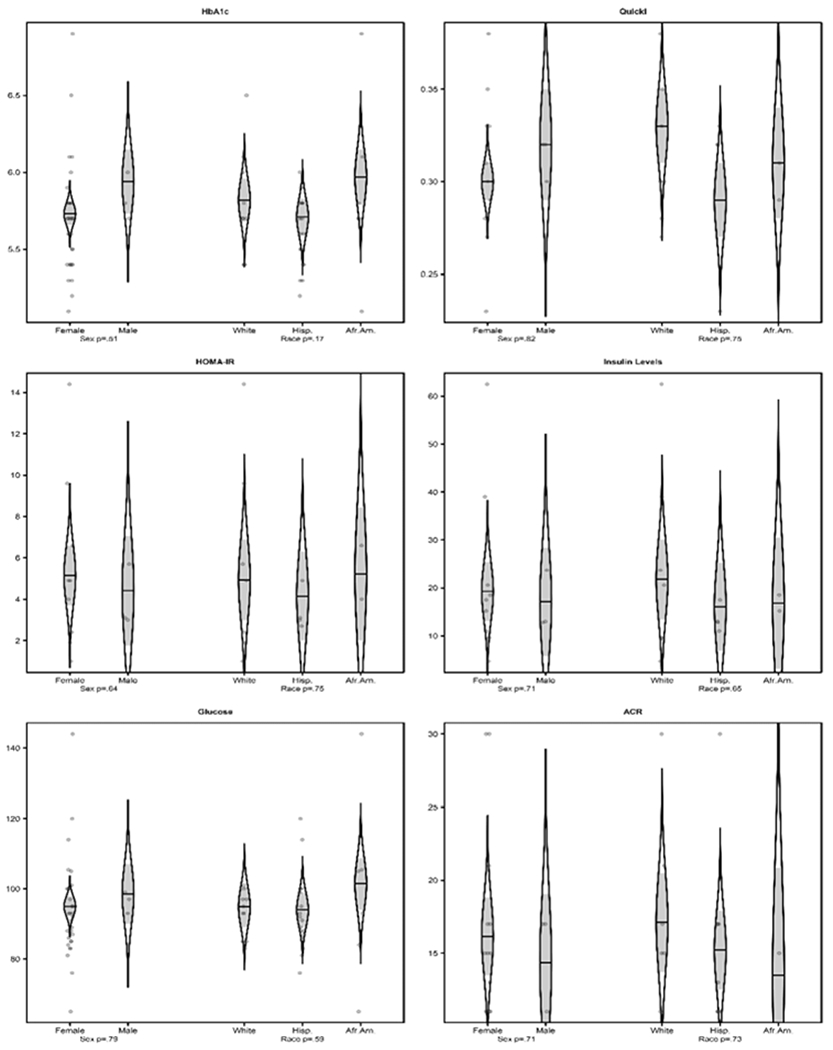
Associations between HbA1c, QUICKI, HOMA-IR, fasting insulin levels, fasting glucose, and ACR FM scores (central pain scores) with gender and ethnicity. Predictions from analysis of variance models are shown as catseye plots (22, 23) which illustrate the normal distribution of the model-adjusted means with shaded +/− standard error intervals, together with scatter plots of the raw measures (randomly jittered horizontally for clarity), and with F-test P-values of associations with gender and ethnicity. There was no significant evidence of an association between the abnormalities discovered with gender or ethnicity.
In 13 patients with FM, we calculated the QUICKI and HOMA-IR indexes. All 13 patients with FM had at least one abnormal value pointing to IR (i.e., QUICKI, or HOMA, or HbA1c). In this subgroup, there were 4 patients with HbA1c below 5.6% (Table 2), but each of these 4 patients showed abnormal QUICKI or HOMA-IR indices, consistent with IR. Conversely, 2 patients with normal QUICKI and HOMA values showed abnormal levels of HbA1c.
Table 2.
Markers in IR in 13 cases of FM.
| Case | Age | G | HbA1c | Insulin | Gluc | QUICKI | HOMA | Central Pain Score |
|---|---|---|---|---|---|---|---|---|
| 1 | 35 | F | 5.3 | 15.2 | 105.4 | 0.31 | 4 | 10 |
| 2 | 51 | F | 5.7 | 4.5 | 93 | 0.38 | 1 | Not avail |
| 3 | 61 | F | 5.8 | 62.5 | 93 | 0.27 | 14.4 | Not avail |
| 4 | 53 | F | 6 | 4.7 | 85 | 0.38 | 1 | 18 |
| 5 | 62 | F | 5.7 | 20.6 | 97 | 0.3 | 4.9 | 15 |
| 6 | 37 | M | 5.8 | 12.8 | 99 | 0.32 | 3.1 | 17 |
| 7 | 55 | F | 5.4 | 39 | 100 | 0.28 | 9.6 | 15 |
| 8 | 41 | F | 5.3 | 17.5 | 114 | 0.23 | 4.9 | 30 |
| 9 | 43 | M | 6 | 13 | 93 | 0.32 | 3 | 17 |
| 10 | 19 | F | 5.4 | 9.4 | 83 | 0.35 | 1.9 | 17 |
| 11 | 43 | F | 6.1 | 16 | 105 | 0.31 | 4.1 | Not avail |
| 12 | 57 | F | 6.9 | 18.5 | 144 | 0.29 | 6.6 | 18 |
| 13 | 65 | M | 5.7 | 23.7 | 97 | 0.3 | 5.7 | 13 |
Values of HbA1c, QUICKI and HOMA-IR in 13 patients with FM. All 13 patients with FM had at least one abnormal value pointing to IR (i.e., QUICKI, HOMA, or HbA1c). In this subgroup, there were 4 patients with HbA1c below 5.6%, but each of these 4 patients showed abnormal QUICKI and HOMA indices, consistent with IR. Conversely, 2 patients with normal QUICKI and HOMA values showed abnormal levels of HbA1c. G: Gender.
Discussion
In this study, we confirmed that most patients afflicted with FM belong to a distinct group that can be separated from 2 control populations by its HbA1c values, a surrogate marker of IR. The introduction of an age adjustment into the linear regression model showed significant differences between patients with FM and control patients. This is important because a large proportion of patients with FM showed HbA1c values considered to be “normal” under current American Diabetes Association (ADA) guidelines (70) (equal or less than 5.6%). However, controlling for the age association with HBA1c disclosed significant differences between the groups (patients with FM versus controls).
The regression relating HbA1c to group (FM HbA1c, FOS NGT, NHANES nondiabetic), summarized in Table 1 and Fig. 1, showed that patients with FM average .54 units of HbA1c higher than FOS NGT, P < .0001, and that patients with FM average .34 units higher than NHANES nondiabetic, P = .0001. It can be visually appreciated in Fig. 1, without sophisticated calculations, that most patients with FM fall at or above the mean of the control patients.
As shown in Table 2, we discovered that by using the QUICKI and HOMA-IR indexes, when assessed along with HbA1c, that 13 of 13 patients with FM (100%) fell outside the normal range currently used in clinical practice for at least one of these 3 tests used in combination (no age correction necessary). In this small sample, all patients with FM showed IR. A caveat that we would like to emphasize is that due to the small number of patients in the subgroup used for the QUICKI AND HOMA-IR calculations, this finding should only be taken as preliminary and as a hypothesis-generating observation. However, if the predictive power of this simple panel (HbA1c, QUICKI, HOMA-IR) is confirmed in larger samples, it may represent a simple, objective laboratory assessment metric in support of a FM diagnosis.
As shown in Fig. 2, analyses of variance relating HbA1c, QUICKI, HOMA-IR, fasting insulin levels, and fasting glucose to gender and ethnicity found no significant evidence of an association, suggesting that the abnormal results are independent of gender and ethnicity. This is another important point because of the higher HbA1c mean values observed in African Americans (71) and Hispanics (72).
Validity of the Markers Used to Identify IR
The “gold standard” to assess IR is the euglycemic clamp method, which produces a steady-state level of exogenous hyperinsulinemia employing a primed and continuous insulin infusion (73). However, this test is cumbersome, time-consuming, and only rarely performed in clinical or research settings. Instead, indirect markers for IR are commonly used, including the HOMA-IR index, QUICKI, HbA1c, and various others. Several investigators reported the QUICKI as one preferred method to measure IR in clinical research settings (74). Briefly, this is an empirical mathematical transformation of fasting blood glucose and plasma insulin that gives reliable and exact assessments of IR (75). It is expressed as the inverse of the sum of the logarithms of the fasting glucose and the fasting insulin level: 1 / (log(fasting insulin μU/mL) + log(fasting glucose mg/dL)). This index correlates closely with the glucose clamp method (71). Values typically associated with the QUICKI are between 0.45 for healthy patients and 0.30 in diabetes (lower numbers indicate higher IR). The HOMA-IR (65), on the other hand, is used to evaluate IR with the formula (fasting serum insulin x glycemia /405). Values of less than 1.0 are considered optimal and above 1.9 indicative of IR.
Measurements of HbA1c, a commonly used determination in clinical practice, correlate closely with other surrogate markers of IR (76). In a study of the relationship between HbA1c and measures of IR, Borai et al (76) assessed the correlation between HbA1c and IR as measured by a variety of different indices in patients from across the glycemic spectrum. Their results indicated that HbA1c is a “simple and reliable marker of IR” even in adults with normal glucose tolerance tests and relatively high insulin sensitivity (76). In another study designed to identify IR among apparently healthy individuals, Saha and Schwarz (77) similarly concluded that “HbA1c is a clinically useful and simple index for predicting the concomitant presence of IR”. Osei et al (78) examined the impact of different levels of HbA1c on insulin sensitivity and found a strong correlation between the HOMA and HbA1c. Specifically, this study reported that the HOMA was significantly correlated with HbA1c (P < 0.01) higher in tertile 3 (3.62 +/− 0.26) than in tertile 1 (2.6 +/− 0.21) of HbA1c and tertile 2 (2.55 +/− 0.31) of HbA1c.
How Could IR Cause FM?
An association does not prove causation. However, it is the first step in generating a hypothesis for further study. With this caveat in mind, we would like to propose the hypothesis that hypoperfusion in the thalamus, insula, and other brain regions associated with the pain experience, contributes to central pain. Prior research has shown that IR leads to dysfunctions in the brain microcirculation, causing cerebral hypoperfusion (34). Interestingly, focal deficits in brain perfusion have been observed in advanced imaging of patients with FM (35). Thus, our working hypothesis is that IR is the missing link in the pathophysiology of FM.
Previous investigations may have overlooked this association because roughly half of the patients with FM exhibit HbA1c levels that are presently considered to be “normal” by current ADA guidelines. This is the first time that HbA1c values are interpreted in the context of patients’ age. Therefore, a value of 5.3% in a younger individual, considered “normal” by current ADA guidelines, may be associated with IR. Additional reasons are that markers of IR, such as the QUICKI and HOMA-IR, are not commonly obtained in the clinical workup of patients with chronic pain or FM.
The association between FM and IR is interesting because it may provide an explanation for central (“brain”) pain mechanisms in other painful disorders (79). FM has also been linked to small fiber neuropathy (SFN), a co-morbidity present in at least 50% of patients with this condition (59,60). Generally, it is difficult to determine the contribution of SFN to the totality of the patients’ pain experience. IR is a frequent cause of peripheral neuropathy and SFN. After reviewing the scientific literature on FM and SFN, we noticed that most of the FM studies did not include a determination of HbA1c levels or other markers of IR. Instead, other less sensitive methods were used (i.e., oral glucose tolerance tests). For example, see Oaklander et al (59).
Importantly, credit should be given to other investigators who have previously proposed similar mechanisms in the pathogenesis of FM. Tishler et al (80) discovered that FM occurred more frequently in patients with diabetes mellitus type 2 than in control patients (18% vs. 2%) and proposed an association between these disorders. Similarly, Yanmaz et al (28) independently published comparable results in patients with FM and diabetes. Fava et al (15) demonstrated that IR was a risk factor for cognitive impairment with FM. However, the abnormalities detected in their published patients were present only in patients with cognitive impairment. An important observation in the study by Fava et al (15) was that the association between IR and the risk of developing cognitive impairment was independent of the body mass index and waist-to-hip ratio of the patients. This suggests that IR in FM may not simply reflect an inconsequential connection with increased body mass index, often present in patients with FM (27). Again, these issues are all worthy of further investigation.
Conclusion
Several neurological disorders are increasingly linked to IR (81–83), and FM may be one additional condition. Our data suggests a tight association between IR and FM, but do not provide information as to which are cause or consequence. Therefore, further research is warranted. Randomized clinical trials of agents that enhance insulin sensitivity in FM should be pursued as the next rational step to advance this hypothesis. In this regard, it will be important to determine if treatment of IR leads not only to improvement in pain scores in FM, but also to correction of brain perfusion abnormalities. Confirmation of such a mechanism may translate into a paradigm shift in FM treatment and result in improved management.
Finally, it is essential to highlight that FM represents a spectrum disorder (79,84,85). Many patients with chronic pain suffer from generalized central (brain) pain, but do not fully meet the current criteria for FM diagnosis (84,85). Preliminary observations indicate that a subgroup of such patients may have IR as an underlying metabolic abnormality. This interesting group of patients has been labeled as having “fibromy-alginess” by experts in this field (84,85).
Acknowledgments
The authors would like to thank the editorial board of Pain Physician for review and criticism in improving the manuscript.
Footnotes
Disclaimer: Dr. Pappolla filed a provisional patent for using anti-diabetic drugs for treatment of fibromyalgia. There was no external funding in the preparation of this manuscript.
Conflict of interest: Each author certifies that he or she, or a member of his or her immediate family, has no commercial association (i.e., consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) that might pose a conflict of interest in connection with the submitted manuscript.
Disclosure
The concept of an association between HbA1c and FM has previously been reported in a small sample of patients (n = 23). Unfortunately, this paper was recently retracted by the journal, based on an equivocal interpretation of an IRB regulation (36). Neither the journal academic editors nor the authors were aware a priori of publication of the regulatory subtleties governing the issues leading to the journal’s decision to retract our paper after publication. For the retracted report, we had obtained a “letter of exemption” before publication. After publication, the journal indicated the LOE should have been obtained prior to initiating data collection (see published retraction notice). The authors disagreed with the retraction notice because the data was always anonymized for the investigators not involved in the direct care of the patients.
References
- 1.Lebovitz HE. Insulin resistance: Definition and consequences. Exp Clin Endocrinol Diabetes 2001; 109:S135–S148. [DOI] [PubMed] [Google Scholar]
- 2.Straub RH. Insulin resistance, selfish brain, and selfish immune system: An evolutionarily positively selected program used in chronic inflammatory diseases. Arthritis Res Ther 2014; 16:S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Joslin EP. The treatment of diabetes mellitus. Can Med Assoc J 1916; 6:673–684. [PMC free article] [PubMed] [Google Scholar]
- 4.Rabinowitch IM. The influence of infection upon the reaction of the diabetic to insulin treatment. Can Med Assoc J 1924; 14:481–482. [PMC free article] [PubMed] [Google Scholar]
- 5.Pemberton R, Foster GL. Studies on arthritis in the Army based on four hundred cases. III. Studies on the nitrogen, urea, carbon dioxid combining power, calcium, total fat and cholesterol of the fasting blood, renal function, blood sugar and sugar tolerance. Arch Int Med 1920; 25:243–282. [Google Scholar]
- 6.Root HF. Insulin resistance and bronze diabetes. N Engl J Med 1929; 201:201–206. [Google Scholar]
- 7.Moller DE, Flier JS. Insulin resistance - mechanisms, syndromes, and implications. N Engl J Med 1991; 325:938–948. [DOI] [PubMed] [Google Scholar]
- 8.Liefmann R. Endocrine imbalance in rheumatoid arthritis and rheumatoid spondylitis; hyperglycemia unresponsiveness, insulin resistance, increased gluconeogenesis and mesenchymal tissue degeneration; preliminary report. Acta Med Scand 1949; 136:226–232. [DOI] [PubMed] [Google Scholar]
- 9.Siva ZO, Uluduz D, Keskin FE, et al. Determinants of glucose metabolism and the role of NPY in the progression of insulin resistance in chronic migraine. Cephalalgia 2018; 38:1773–1781. [DOI] [PubMed] [Google Scholar]
- 10.Greisen J, Juhl CB, Grøfte T, Vilstrup H, Jensen TS, Schmitz O. Acute pain induces insulin resistance in humans. Anesthesiology 2001; 95:578–584. [DOI] [PubMed] [Google Scholar]
- 11.Zhai X, Sun C, Rong P, et al. A correlative relationship between chronic pain and insulin resistance in Zucker fatty rats: Role of downregulation of insulin receptors. J Pain 2016; 17:404–413. [DOI] [PubMed] [Google Scholar]
- 12.Ray L, Lipton RB, Zimmerman ME, Katz MJ, Derby CA. Mechanisms of association between obesity and chronic pain in the elderly. Pain 2011; 152:53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.García G, Gutiérrez-Lara EJ, Centurión D, Granados-Soto V, Murbartián J. Fructose-induced insulin resistance as a model of neuropathic pain in rats. Neuroscience 2019; 404:233–245. [DOI] [PubMed] [Google Scholar]
- 14.Bosco D, Plastino M, Cristiano D, et al. dementia is associated with insulin resistance in patients with Parkinson’s disease. J Neurol Sci 2012; 315:39–43. [DOI] [PubMed] [Google Scholar]
- 15.Fava A, Plastino M, Cristiano D, et al. Insulin resistance possible risk factor for cognitive impairment in fibromialgic patients. Metab Brain Dis 2013; 28:619–627. [DOI] [PubMed] [Google Scholar]
- 16.Merskey H, Bogduk N. Classification of chronic pain: Descriptions of chronic pain syndromes and definition of pain terms. 2nd ed. Task Force on Taxonomy of the International Association for the Study of Pain. IASP Press, Seattle, 1994. [Google Scholar]
- 17.Smith HS, Harris R, Clauw D. Fibromyalgia: An afferent processing disorder leading to a complex pain generalized syndrome. Pain Physician 2011; 14:E217–E245. [PubMed] [Google Scholar]
- 18.Arroyo-Fernandez R, Bravo-Esteban E, Domenech-Garcia V, Ferri-Morales A. Pressure-induced referred pain as a biomarker of pain sensitivity in fibromyalgia. Pain Physician 2020; 23:E353–E362. [PubMed] [Google Scholar]
- 19.Coppieters I, Ickmans K, Cagnie B, et al. Cognitive performance is related to central sensitization and health-related quality of life in patients with chronic whiplash-associated disorders and fibromyalgia. Pain Physician 2015; 18:E389–E401. [PubMed] [Google Scholar]
- 20.Ickmans K, Meeus M, De Kooning M, Lambrecht L, Pattyn N, Nijs J. Associations between cognitive performance and pain in chronic fatigue syndrome: co-morbidity with fibromyalgia does matter. Pain Physician 2015; 18:E841–E852. [PubMed] [Google Scholar]
- 21.Goubert D, Danneels L, Graven-Nielsen T, Descheemaeker F, Meeus M. Differences in pain processing between patients with chronic low back pain, recurrent low back pain, and fibromyalgia. Pain Physician 2017; 20:307–318. [PubMed] [Google Scholar]
- 22.Thorp SL, Suchy T, Vadivelu N, Helander EM, Urman RD, Kaye AD. Functional connectivity alterations: Novel therapy and future implications in chronic pain management. Pain Physician 2018; 21:E207–E214. [PubMed] [Google Scholar]
- 23.Wolfe F, Ross K, Anderson J, Russell IJ. Aspects of fibromyalgia in the general population: Sex, pain threshold, and fibromyalgia symptoms. J Rheumatol 1995; 22:151–156. [PubMed] [Google Scholar]
- 24.Wolfe F, Ross K, Anderson J, Russell IJ, Hebert L. The prevalence and characteristics of fibromyalgia in the general population. Arthritis Rheum 1995; 38:19–28. [DOI] [PubMed] [Google Scholar]
- 25.Svetvold H, Stiles TC, Landro NI. Information processing in primary fibromyalgia, major depression and healthy controls. J Rheumatol 1995; 22:137–142. [PubMed] [Google Scholar]
- 26.Leavitt F, Katz RS. Distraction as a key determinant of impaired memory in patients with fibromyalgia. J Rheumatol 2006; 33:127–132. [PubMed] [Google Scholar]
- 27.Okifuji A, Bradshaw DH, Olson C. Evaluating obesity in fibromyalgia: Neuroendocrine biomarkers, symptoms, and functions. Clin Reumatol 2009; 28:475–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yanmaz MN, Mert M, Korkmaz M. The prevalence of fibromyalgia syndrome in a group of patients with diabetes mellitus. Rheumatol Int 2012; 32:871–874. [DOI] [PubMed] [Google Scholar]
- 29.Loevinger BL, Muller D, Alonso C, Coe CL. Metabolic syndrome in women with chronic pain. Metabolism 2007; 56:87–93. [DOI] [PubMed] [Google Scholar]
- 30.Ott A, Stolk RP, Van Harskamp F, Pols HA, Hofman A, Breteler MM. Diabetes mellitus and the risk of dementia: The Rotterdarm Study. Neurology 1999; 53:1937–1942. [DOI] [PubMed] [Google Scholar]
- 31.Xu WL, Qiu CX, Wahlin A, Winblad B, Fratiglioni L. Diabetes mellitus and risk of dementia in the Kungshol men project: A 6-year follow-up study. Neurology 2004; 63:1181–1186. [DOI] [PubMed] [Google Scholar]
- 32.Luchsinger JA, Tang MX, Shea S, Mayeux R. Hyperinsulinemia and risk of Alzheimer’s disease. Neurology 2004; 63:1187–1192. [DOI] [PubMed] [Google Scholar]
- 33.Bosco D, Fava A, Plastino M, Montalcini T, Pujia A. Possible implications of insulin resistance and glucose metabolism in Alzheimer’s disease pathogenesis. J Cell Mol Med 2011; 15:1807–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Frosch OH, Yau PL, Osorio RS, Rusinek H, Storey P, Convit A. Insulin resistance among obese middle-aged is associated with decreased cerebrovascular reactivity. Neurology 2017; 89:249–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mountz JM, Bradley LA, Modell JG, et al. Fibromyalgia in women. Abnormalities of regional cerebral blood flow in the thalamus and the caudate nucleus are associated with low pain threshold levels. Arthritis Rheum 1995; 38:926–938. [DOI] [PubMed] [Google Scholar]
- 36.Pappolla MA, Manchikanti L, Andersen CR, et al. Is insulin resistance the cause of fibromyalgia? A preliminary report. PLoS One 2019; 14:e0216079 (Retracted 2019; 14:e0226174). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 37.Queiroz LP. Worldwide epidemiology of fibromyalgia. Curr Pain Headache Rep 2013; 17:356. [DOI] [PubMed] [Google Scholar]
- 38.Croft P, Rigby AS, Boswell R, Schollum J, Silman A. The prevalence of chronic widespread pain in the general population.J Rheumatol 1993; 20:710–713. [PubMed] [Google Scholar]
- 39.Rencber N, Saglam G, Huner B, Kuru O. Presence of fibromyalgia syndrome and its relationship with clinical parameters in patients with axial spondyloarthritis. Pain Physician 2019; 22:E579–E585. [PubMed] [Google Scholar]
- 40.Ruiz-Montero PJ, Segura-Jimenez V, Alvarez-Gallardo IC, et al. fibromyalgia impact score in women with fibromyalgia across Southern, Central, and Northern Areas of Europe. Pain Physician 2019; 22:E511–E516. [PubMed] [Google Scholar]
- 41.Chen JH, Chen HJ, Kao CH, Tseng CH, Tsai CH. Is fibromyalgia risk higher among male and young inflammatory bowel disease patients? Evidence from a Taiwan cohort of one million. Pain Physician 2018; 21:E257–E264. [PubMed] [Google Scholar]
- 42.Coppieters I, Cagnie B, Nijs J, et al. Effects of stress and relaxation on central pain modulation in chronic whiplash and fibromyalgia patients compared to healthy controls. Pain Physician 2016; 19:119–130. [PubMed] [Google Scholar]
- 43.Thorpe J, Shum B, Moore RA, Wiffen PJ, Gilron I. Combination pharmacotherapy for the treatment of fibromyalgia in adults. Cochrane Database Syst Rev 2018; 2:CD010585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schulze NB, Salemi MM, de Alencar GG, Moreira MC, de Siqueira GR. Efficacy of manual therapy on pain, impact of disease, and quality of life in the treatment of fibromyalgia: A systematic review. Pain Physician 2020; 23:461–476. [PubMed] [Google Scholar]
- 45.Salazar AP, Stein C, Marchese RR, Plentz RD, Pagnussat AS. electric stimulation for pain relief in patients with fibromyalgia: a systematic review and meta-analysis of randomized controlled trials. Pain Physician 2017; 20:15–25. [PubMed] [Google Scholar]
- 46.Castro-Sanchez AM, Garcia-Lopez H, Mataran-Penarrocha GA, et al. Effects of dry needling on spinal mobility and trigger points in patients with fibromyalgia syndrome. Pain Physician 2017; 20:37–52. [PubMed] [Google Scholar]
- 47.Yeh SW, Hong CH, Shih MC, Tam KW, Huang YH, Kuan YC. Low-level laser therapy for fibromyalgia: A systematic review and meta-analysis. Pain Physician 2019; 22:241–254. [PubMed] [Google Scholar]
- 48.Dieleman JL, Baral R, Birger M, et al. US spending on personal health care and public health, 1996-2013. JAMA 2016; 316:2627–2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dieleman JL, Cao J, Chapin A, et al. US health care spending by payer and health condition, 1996-2016. JAMA 2020; 323:863–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Talotta R, Bazzichi L, Di Franco M, et al. One year in review 2017: Fibromyalgia. Clin Exp Rheumatol 2017; 35:6–12. [PubMed] [Google Scholar]
- 51.Silverman S, Dukes EM, Johnston SS, Brandenburg NA, Sadosky A, Huse DM. The economic burden of fibromyalgia: Comparative analysis with rheumatoid arthritis. Curr Med Res Opin 2009; 25:829–840. [DOI] [PubMed] [Google Scholar]
- 52.Leadley RM, Armstrong N, Lee YC, Allen A, Kleijnen J. Chronic diseases in the European Union: The prevalence and health cost implications of chronic pain. J Pain Palliat Care Pharmacother 2012; 26:310–325. [DOI] [PubMed] [Google Scholar]
- 53.Boomershine CS. Fibromyalgia: The prototypical central sensitivity syndrome. Curr Rheumatol Rev 2015; 11:131–145. [DOI] [PubMed] [Google Scholar]
- 54.Ablin JN, Buskila D. Update on the genetics of the fibromyalgia syndrome. Best Pract Res Clin Rheumatol 2015; 29:20–28. [DOI] [PubMed] [Google Scholar]
- 55.Ablin JN, Bar-Shira A, Yaron M, Orr-Urtreger A. Candidate-gene approach in fibromyalgia syndrome: Association analysis of the genes encoding substance P receptor, dopamine transporter and alpha1-antitrypsin. Clin Exp Rheumatol 2009; 27:S33–S38. [PubMed] [Google Scholar]
- 56.Staud R. Cytokine and immune system abnormalities in fibromyalgia and other central sensitivity syndromes. Curr Rheumatol Rev 2015; 11:109–115. [DOI] [PubMed] [Google Scholar]
- 57.Dell’Osso L, Bazzichi L, Baroni S, et al. The inflammatory hypothesis of mood spectrum broadened to fibromyalgia and chronic fatigue syndrome. Clin Exp Rheumatol 2015; 33:S109–S116. [PubMed] [Google Scholar]
- 58.Wolfe F, Clauw DJ, Fitzcharles MA, et al. 2016 revisions to the 2010/2011 fibromyalgia diagnostic criteria. Semin Arthritis Rheum 2016; 46:319–329. [DOI] [PubMed] [Google Scholar]
- 59.Oaklander AL, Herzog ZD, Downs HM, Klein MM. Objective evidence that small-fiber polyneuropathy underlies some illnesses currently labeled as fibromyalgia. Pain 2013; 154:2310–2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Grayston R, Czanner G, Elhadd K, et al. A systematic review and meta-analysis of the prevalence of small fiber pathology in fibromyalgia: Implications for a new paradigm in fibromyalgia etiopathogenesis. Semin Arthritis Rheum 2019; 48:933–940. [DOI] [PubMed] [Google Scholar]
- 61.Pani LN, Korenda L, Meigs JB, et al. Effect of aging on A1C levels in individuals without diabetes: Evidence from the Framingham Offspring Study and the National Health and Nutrition Examination Survey 2001-2004. Diabetes Care 2008; 31:1991–1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thompson BM, Scott BI, Boiani JA. Understanding the Food and Drug Administration’s jurisdiction over laboratory-developed tests and divisions between food, drug, and cosmetic act-regulated and clinical laboratory improvement amendments of 1988-regulated activities. Clin Lab Med 2016; 36:575–585. [DOI] [PubMed] [Google Scholar]
- 63.Mahmood SS, Levy D, Vasan RS, Wang TJ. The Framingham Heart Study and the epidemiology of cardiovascular disease: A historical perspective. Lancet 2014; 383:999–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Katz A, Nambi SS, Mather K, et al. Quantitative insulin sensitivity check index: A simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab 2000; 85:2402–2410. [DOI] [PubMed] [Google Scholar]
- 65.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985; 28:412–419. [DOI] [PubMed] [Google Scholar]
- 66.Tukey JW. Comparing individual means in the analysis of variance. Biometrics 1949; 5:99–114. [PubMed] [Google Scholar]
- 67.Akaike H. A new look at the statistical model identification. IEEE Transactions on Automatic Control 1974; 19:716–723. [Google Scholar]
- 68.Cumming G. The new statistics: Why and how. Psychol Sci 2014; 25:7–29. [DOI] [PubMed] [Google Scholar]
- 69.Andersen CR. Catseyes: Create catseye plots illustrating the normal distribution of the means. R package version 0.2.5 Accessed 12/1/2020. https://rdrr.io/cran/catseyes/ [Google Scholar]
- 70.American Diabetes Association. 2. Classification and diagnosis of diabetes: Standards of medical care in diabetes-2020. Diabetes Care 2020; 43:S14–S31. [DOI] [PubMed] [Google Scholar]
- 71.Kirk JK, D’Agostino RB Jr., Bell RA, et al. Disparities in HbA1c levels between African-American and non-Hispanic white adults with diabetes: A meta-analysis. Diabetes Care 2006; 29:2130–2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Avilés-Santa ML, Hsu LL, Arredondo M, et al. Differences in hemoglobin A1c between Hispanics/Latinos and Non-Hispanic Whites: An analysis of the Hispanic Community Health Study/Study of Latinos and the 2007-2012 National Health and Nutrition Examination Survey. Diabetes Care 2016; 39:1010–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: A method for quantifying insulin secretion and resistance. Am J Physiol 1979; 237:E214–E223. [DOI] [PubMed] [Google Scholar]
- 74.Chen H, Sullivan G, Quon MJ. Assessing the predictive accuracy of QUICKI as a surrogate index for insulin sensitivity using a calibration model. Diabetes 2005; 54:1914–1925. [DOI] [PubMed] [Google Scholar]
- 75.Muniyappa R, Madan R. Assessing insulin sensitivity and resistance in humans. 2018 Jul 10. In; Feingold KR, Anawalt B, Boyce A, et al. (eds). Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000. –. [Google Scholar]
- 76.Borai A, Livingstone C, Abdelaal F, Bawazeer A, Keti V, Ferns G. The relationship between glycosylated haemoglobin (HbA1c) and measures of insulin resistance across a range of glucose tolerance. Scand J Clin Lab Invest 2011; 71:168–172. [DOI] [PubMed] [Google Scholar]
- 77.Saha S, Schwarz PEH. Impact of glycated hemoglobin (HbA1c) on identifying insulin resistance among apparently healthy individuals. J Public Health 2017; 25:505–512. [Google Scholar]
- 78.Osei K, Rhinesmith S, Gaillard T, Schuster D. Is glycosylated hemoglobin A1c a surrogate for metabolic syndrome in non-diabetic, first-degree relatives of African-American patients with type 2 diabetes? J Clin Endocrinol Metab 2003; 88:4596–4601. [DOI] [PubMed] [Google Scholar]
- 79.Aoyagi K, He J, Nicol AL, et al. A subgroup of chronic low back pain patients with central sensitization. Clin J Pain 2019; 35:869–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tishler M, Smorodin T, Vazina-Amit M, Ramot Y, Koffler M, Fishel B. Fibromyalgia in diabetes mellitus. Rheumatol Int 2003; 23:171–173. [DOI] [PubMed] [Google Scholar]
- 81.Talbot K, Wang HY, Kazi H, et al. Demonstrated brain insulin resistance in Alzheimer’s disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J Clin Invest 2012; 122:1316–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Athauda D, Foltynie T. Insulin resistance and Parkinson’s disease: A new target for disease modification? Prog Neurobiol 2016; 145–146:98–120. [DOI] [PubMed] [Google Scholar]
- 83.Watson K, Nasca C, Aasly L, McEwen B, Rasgon N. Insulin resistance, an unmasked culprit in depressive disorders: Promises for interventions. Neuropharmacology 2018; 136:327–334. [DOI] [PubMed] [Google Scholar]
- 84.Brummett CM, Goesling J, Tsodikov A, et al. Prevalence of the fibromyalgia phenotype in patients with spine pain presenting to a tertiary care pain clinic and the potential treatment implications. Arthritis Rheum 2013; 65:3285–3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Brummett CM, Janda AM, Schueller CM, et al. Survey criteria for fibromyalgia independently predict increased postoperative opioid consumption after lower-extremity joint arthroplasty: A prospective, observational cohort study. Anesthesiology 2013; 119:1434–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]


