Abstract
Aims
Over the past 25 years there has been a substantial development in the field of digital electrophysiology (EP) and in parallel a substantial increase in publications on digital cardiology.
In this celebratory paper, we provide an overview of the digital field by highlighting publications from the field focusing on the EP Europace journal.
Results
In this journey across the past quarter of a century we follow the development of digital tools commonly used in the clinic spanning from the initiation of digital clinics through the early days of telemonitoring, to wearables, mobile applications, and the use of fully virtual clinics. We then provide a chronicle of the field of artificial intelligence, a regulatory perspective, and at the end of our journey provide a future outlook for digital EP.
Conclusion
Over the past 25 years Europace has published a substantial number of papers on digital EP, with a marked expansion in digital publications in recent years.
Keywords: Digital, Telemonitoring, Artificial intelligence
What’s New?
A comprehensive overview of the past 25 years within the field of digital electrophysiology with a particular focus on publications from the EP Europace journal.
Introduction
Digital technology has the potential to impact and transform healthcare by providing a platform for patient identification, risk stratification, management, patient interactivity, and education. In the past 25 years there has been a substantial development within the field of digital cardiology, with electrophysiology (EP) in the forefront paralleled by an increase in publications within this field.
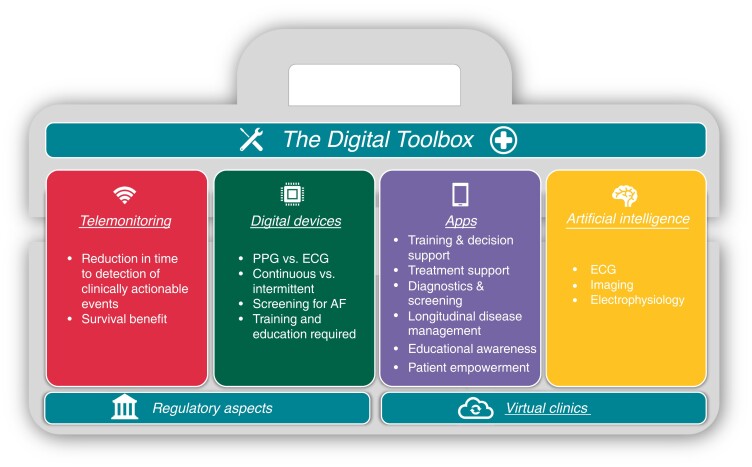
This paper seeks to provide an in-depth overview and chronology of the field of digital EP mirroring the substantial influence in this area that EP Europace has had in the past decades. Digital technologies can enable care for arrhythmia patients, and we aim to provide the reader with a brief overview of the digital toolbox, starting with a journey from the early days of telemonitoring, followed by an update on monitoring from a wearable perspective. From there we move forward to mobile applications and virtual clinics. In addition, a chronicle of the development within the field of artificial intelligence (AI), an outlook on the future as well as a regulatory perspective, is provided (Figure 1).
Figure 1.
An overview of digital tools available for the electrophysiologist.
Telemonitoring of cardiac implantable electronic devices
The digital journey in EP begins with cardiac implantable electronic devices (CIEDs). A significant evolution has been seen over the last decade, with novel technologies allowing complex programming and wireless remote monitoring (RM) of device function and patient health status.1–6 Telemonitoring of CIEDs has now evolved to a fully automated system to complement in-office follow-up7 and has gained even more importance during the COVID-19 pandemic.8
Previous studies have demonstrated a reduction in time to detection of clinically actionable events, prompting earlier intervention with the implementation of RM compared to standard in-person follow-up care.9–14 In the multicenter RIONI study, in 619 patients with an implantable cardioverter-defibrillator (ICD) it was shown that home monitoring could provide an accurate evaluation of events by experts.5,15 The PREFER study evaluated 980 pacemaker patients with RM providing earlier and more frequent detection of clinically relevant events.9 The TRUST study showed a median time to evaluation after an arrhythmic event of less than 2 days, compared to 36 days in the control group.16,17 Access to continuous RM data has resulted in fewer in-person evaluations, a reduction in emergent and unscheduled hospital visits with a decrease in overall healthcare utilization.6,16,18–22 However, apart from the COMPAS trial, most of these studies were conducted only in ICD patients. Prompt arrhythmia detection coupled with early recognition of fluid accumulation23 with an in-built algorithm reduced emergency department and urgent in-office visits by 35% in the remote arm, as demonstrated by the EVOLVO study.18 Other large-scale studies have shown the potential cost saving associated with RM strategy.21,22,24–30
More importantly, a pooled analysis of three randomized controlled trials (TRUST, ECOST, and IN-TIME) involving 2405 patients with ICDs showed a significant reduction of all-cause mortality with RM.31 Another meta-analysis of nine randomised controlled trials (RCTs) demonstrated non-inferiority of RM and in-office follow-up with RCTs utilizing daily transmission verification, proving significant survival benefit.32 Large real-world registries have further established the survival benefit of RM also emphasizing the impact of adherence to RM in improving patient outcomes.29,33–35
Despite its various proven clinical benefits, RM implementation and uptake have been modest.36 Barriers to RM implementation are multifactorial and include patient factors such as health literacy, preference and access, lack of healthcare infrastructure, and inadequate reimbursement.37 The Altitude Survival Study found that more than 60% of patients with RM-capable devices did not participate in RM.35 Real-world population studies also revealed poor compliance to RM, with one study reporting 53% of patients without a single RM transmission over the follow-up period, and another with 21% non-compliance rate.33,38
On the other hand, the increasing volume of RM transmissions has reached a staggering proportion and increased the clinic workloads. In a study involving more than 26 000 patients, the number of transmissions and alert burden was quantified, resulting in a total of 205 804 transmissions, 40% of which were alert with only 4.8% requiring urgent clinical response.39 This data deluge, which includes a high rate of false positives, particularly with the increasing use of implantable loop recorders, leads to an increased burden on clinical staff, and delays in the evaluation of actionable alerts.40,41 Early studies suggested how this may be partially overcome using AI to better identify actionable alerts.42 However, critical for improved patient outcomes are the clinic-level pathways to manage actionable alerts. Evidence suggests this may pose a significant threat to the success of RM.43 Ultimately, patient education, streamlined alert settings and clinic workflow, adequate trained staffing, and attractive reimbursement policies must be in place to ensure successful adoption of the RM approach for CIEDs.44,45 Recently, a query has been raised if smartwatches can provide a replacement for CIEDs, which will be addressed in the next section.46
Digital devices
In concordance with telemonitoring, cardiac rhythm monitoring has also markedly evolved in the past 25 years, progressing from the initial Holter monitors to event recorders, mobile cardiac telemetry, implantable cardiac monitors to increasingly ‘smart’ multipurpose sensing and monitoring instruments.47,48
Wearable devices have become central for cardiac rhythm monitoring. In 1994, the first wrist-worn heart rhythm monitor was introduced. This device was capable of transmitting an analogue transtelephonic signal that was converted into a digitized single-lead ECG tracing. Unfortunately, adoption was limited as patients found the device more difficult to use than traditional Holter monitors.49 Several other wrist-worn devices and simple textile-based heart rate monitors soon followed.50 While they provided some insight into a patient’s HR, they were not accurate enough for clinical use and were primarily used by fitness enthusiasts. In the last decade, advances in wearable technology have led to more accurate and reliable devices for cardiac rhythm monitoring.51 Most of these devices, such as smartwatches, rings, and fitness trackers, use optical sensors to detect the patient’s HR and rhythm using photoplethysmography, providing real-time monitoring and analysis.52
More modern wearable devices, such as smartwatches, use either photoplethysmography and/or ECG-based HR and rhythm monitoring providing single-lead ECGs or even multiple lead ECGs. These devices can also track other metrics such as physical activity, sleep, and stress levels, providing a more holistic view of the patient’s health.
Digital devices offer new possibilities for continuous or intermittent monitoring and have been increasingly used for screening for atrial fibrillation (AF).53,54 Large-scale studies in different settings and populations have shown that screening for AF using digital devices identifies patients at risk,55–59 has the potential to reduce relevant outcomes60 and reduce costs.61 Screening for AF using new digital devices is recommended in guidelines and consensus documents.51,62,63
Clinical usage and acceptance of digital devices have increased in the last few years. The true advantages of digital care were seen during the time of the COVID-19 pandemic when digital devices still allowed specific and dedicated remote patient care for arrhythmia management, as shown in the international TELECHECK-AF project.64–68 Healthcare providers have since started to recognize the potential benefits of these devices for patient care, and digitally advanced centres are using them as a tool for remote patient monitoring.
While wearable devices have shown promise in cardiac rhythm monitoring, there are still challenges that need to be addressed. In particular, the accuracy and reliability of wearable devices for cardiac rhythm and rate monitoring have been a subject of debate.69,70 Recent studies have shown that although some wearable devices can provide reliable measurements of HR and rhythm, variations due to the manufacturer’s algorithms and the patient population are common.71 Another challenge is the interpretation of the data generated by these devices, which can be complex and requires specialized knowledge.72,73 As recently shown in an European Heart Rhythm Association (EHRA) survey, digital devices are widely used, but reimbursement for usage and interpretation is a problem to solve in most countries.74 In the future, we can expect to see further advances in wearable technology for cardiac rhythm monitoring.75 These devices may become more accurate, reliable, and personalized to the patient's needs, and advances in AI may enable more efficient and accurate interpretation of the generated data.76 In addition, patient involvement remains an important aspect of digital care.77,78
In conclusion, wearable devices have evolved significantly in the last 25 years and have the potential to revolutionize cardiac rhythm monitoring. While there are still some challenges that need to be addressed, they have shown promise in improving patient outcomes through earlier detection and treatment of cardiac conditions. As technology continues to advance, we can expect to see further improvements in cardiac rhythm monitoring. Many wearables are connected to mobile health applications, which will be discussed in the coming section.
Mobile health applications (apps)
The introduction of novel generations of smartphones using computer-like built-in features and sensors on the market in 2007, allowed for customization of the devices by downloading apps from central stores. This feature, combined with the high-grade adoption of smartphone technology in the population (i.e. 86% penetration rate in Europe in 2021) increases the possible applications in the EP field.79
In EP, the initial interest with regards to smartphones was focused on safety, in particular by determining the potential interference of smartphones with implantable cardioverter defibrillators.80
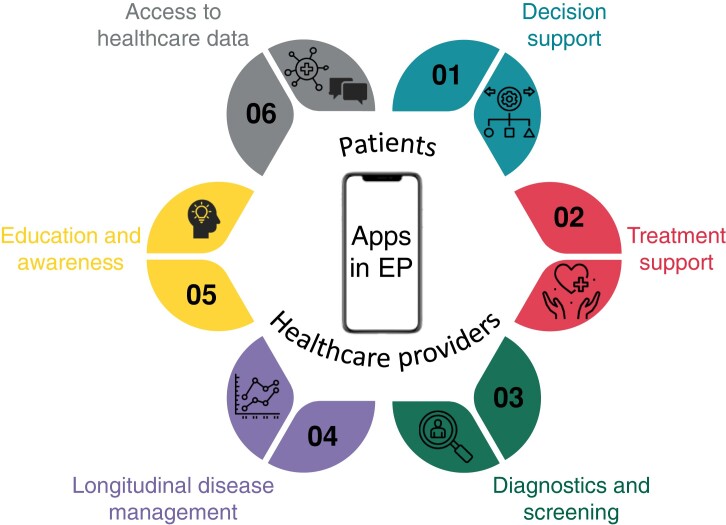
More recently, evaluation studies comparing the accuracy of handheld connected devices and smartphone apps have gained a lot of attention.81 The use of apps within healthcare has manifold opportunities when implemented in a structured pathway, as described in multiple publications (Figure 2).
Figure 2.
Overview of opportunities in mobile phone applications in electrophysiology.
Training of healthcare providers and decision support. Providing care that conforms to the guidelines is of pivotal importance to optimize patient outcomes, but adherence to the guidelines is often suboptimal. The availability of interactive clinical practice guidelines through an app, such as the ‘ESC Pocket Guidelines’ app, could facilitate their uptake. On top of this, for AF the CATCH ME Consortium developed the ‘AF Manager’ app as a tool in which healthcare professionals can incorporate patient data to suggest treatment options that conform to the guidelines.82,83
Treatment support. To support patients in their daily treatment and to promote a healthy lifestyle, mHealth apps can enhance adherence to medication, increase adherence to hospital appointments, support patients in rehabilitation and physical activity and assist in tackling of comorbidities.84–88
Diagnostics and screening. Various apps can be used to screen for arrhythmias making use of different sensors embedded in the smartphone or connected to it.51,71
Longitudinal disease management. mHealth opens a large spectrum for the (remote) follow-up of various clinical parameters to fill in the gaps between the in-person consultation visits, including HR, heart rhythm, symptoms, weight, and blood pressure.51,67,68,83,84,87,89–91 Apps can also connect with wearables, or with implanted devices to collect valuable clinical information on the patient’s status.84,92–94
Education and awareness. To engage patients in their own care and allow shared decision-making, mHealth apps can assist in delivering validated information and tailored education to patients to increase health literacy.82,87,95,96
Empowering patients to own their health data and directly contact healthcare providers. Apps can allow patients to get informed about their healthcare data and contact their healthcare providers in case of questions about their management. Moreover, many hospitals have their own applications allowing patients to counsel their health data.
Despite the fact that mHealth apps are widely available, several barriers93,97,98 still exist. These include in particular, lack of validation, sparse data on effectiveness and impact on clinically relevant endpoints, poor data integration with electronic health record systems, lack of clear guidance on care pathways to make use of these apps in daily clinical practice, and lack of reimbursement.
Studies formally evaluating the impact of mHealth apps on healthcare professional’s behaviour are scarce and larger-scale studies with representative patient cohorts, appropriate comparators, and longer-term assessment of the impact of mHealth apps are warranted, also in view of the new requirements for conformity assessment introduced by the EU Medical Device Regulation.87,99 As a result, apps are rarely prescribed to patients by healthcare providers in daily clinical practice.
The use of apps for medical purposes could further expand in the future when current barriers in the development, security, validation, cost-effectiveness, interoperability, implementation, and reimbursement of mHealth in daily clinical practice will be solved, and it is an integral part of virtual clinics.97,98
Virtual clinics
As highlighted in the previous sections the publication of ‘Transtelephone Pacemaker Clinic’ in 1971 and the subsequent rise of RM of CIEDs established cardiac EP as leaders in providing virtual care for patients.100 The rapid transition to virtual modalities to provide safe, uninterrupted arrhythmia care during the COVID-19 pandemic led to an exponential adoption of digital care by EP.101–103 This cemented the view of EPs as one of the highest adopters of virtual care (>95% in some systems), and a high rate of virtual care is maintained even after the pandemic has largely subsided.65,66,104
EP is particularly conducive to the adoption of virtual clinics as most consultations can be performed entirely virtually. Cardiac rhythm tracings can all be reviewed and analysed online. Discussions, including shared decision-making, can be performed via video or telephone contact.102 HR and rhythm data from direct-to-consumer digital devices continue to be integrated, helping to enrich arrhythmia patient virtual care.51,65,93,105
Real-world studies have shown feasibility, safety, and efficacy of virtual arrhythmia clinics, with similar outcomes, quality metrics, and patient satisfaction when compared to in-person visits.106–108 Virtual AF management has shown particular promise. One of the largest endeavours has been the TELECHECK-AF virtual clinic.68,91 Here, teleconsultation, use of a CE-certified, clinically validated smartphone photoplethysmography HR and rhythm monitoring App (FibriCheck, Flanders, Belgium), and virtual education were combined to support comprehensive AF management.68,109 Patients used the app to check HR/rhythm three times a day for one week, and data was uploaded to the cloud for clinician review before teleconsultation.68 In 20 days after launch, 9 countries/23 European centers adopted this virtual clinic model; by 6 months nearly 1700 patients were enrolled.52,110 Patients have found the app easy to use (94%), providing them a sense of reassurance (74%); clinicians have given high ratings for on-boarding, cloud access, and reliability.67 Currently, over 6000 AF patients have received care via this virtual clinic model. The TeleWAS-AF ‘wait-and-see’ programme for patients with recently diagnosed AF uses this same virtual care strategy to help avoid unnecessary cardioversions.67 A randomized trial, RACE 9 OBSERVE-AF, assessing this virtual care pathway is currently underway (clinicaltrials.gov NCT04612335). In the UK, an ‘AF virtual ward’ has recently been piloted to manage hemodynamically stable AF patients in an ambulatory setting by using digital tools for vital signs monitoring (hand-held daily ECGs, BP monitoring, and O2 saturations), twice daily ‘virtual’ rounds, and medication adjustments via a clinical pharmacy. This proof-of-concept study recently showed potential for decreasing AF hospital admissions and re-admissions.111
Virtual clinics for the management of anticoagulation have been well-established.112,113 Virtual clinics for outpatient antiarrhythmic drug loading have been less explored. In an initial feasibility study, three patients with CIEDs requiring sotalol initiation during the COVID-19 pandemic were monitored from home via CIED remote transmissions, mobile cardiac telemetry, a hand-held 6L ECG device Food and Drug Adminstration-cleared for QTc monitoring (KardiaMobile® 6L, Alivecor, Mountainview, CA), as well as video-telehealth. Successful outpatient initiation of sotalol initiation was performed without any adverse events.114A pharmacist-driven virtual clinic for outpatient sotalol loading and monitoring has since been safely piloted using online ECG and lab review, telephone contact, and remote QTc monitoring via the KardiaMobile® 6L.115
Virtual clinics for post-AF ablation patients show potential. One study on 46 AF patients from the UK replaced the 3-month post-ablation in-person visit with a video visit coupled with a proprietary vital sign tracking mApp. This virtual clinic showed high overall patient satisfaction (84%) and patient cost and time savings (80%).116 The Cleveland Clinic ‘Atrial Fibrillation Future Clinic’ randomized 100 post-ablation patients to traditional in-person care vs. virtual care enhanced with a hand-held ECG monitor and follow-up at 6 months. Hospitalization, ER, and clinic visits, as well as anxiety, were similar between groups. In addition, the virtual care group had less use of ambulatory ECGs.117
As virtual clinics and digital devices are further integrated into EP, patient perspectives—preferences, readiness, digital access, availability, and literacy, as well as cost—must be considered.51,75 Canadian studies have shown that arrhythmia patient virtual care may be well received for quality of life, cost and time savings, and opportunities for participation from caregivers and family members.118,119 However, it may be less preferred for new patients or complex issues requiring nuanced discussions. Fit (or non-fit) of virtual clinics has been found to be dependent on clinician and medical staff’s ability to communicate via these channels effectively and comprehensively.119 Hybrid models combining in-person with virtual clinics may be an effective middle-ground for both patients and clinicians. Also, AI might have a future role in virtual clinics, by ECG interpretation and prediction of outcomes.
Artificial intelligence
AI and machine learning (ML) are rapidly evolving disciplines within data science that can classify complex data, and thus ‘interpret them’ to predict future patterns or risk of events.120 Studies published in Europace within the field of AI provide an exciting chronology of our field aimed at better-managing patients with cardiac electrophysiologic disorders.
Europace published its first AI study in 2003, well before its 25th birthday, in which Kappenberger et al.121 identified ventricular tachycardia (VT)/ventricular fibrillation (VF) with a c-statistic >0.90 by leveraging sensed voltage alterations from sinus rhythm in ICD recipients. This early study incorporated elements that remain foundational to this day, most notably separating the cohorts used for algorithm development from cohorts used for testing to improve the generalizability of results. Studies in 2008 used AI of electrogram shapes to discriminate VT with such high accuracy (c-statistic > 0.95)122 that an accompanying editorial123 posed a question that still resonates: ‘[will] automated analysis … replace the electrophysiologist?’.
Of numerous studies using AI to predict VT/VF, Shakibfar et al used random forests to classify daily ICD interrogation summaries in 19 935 patients, providing a c-statistic of 0.80 for imminent electrical storm in an independent test cohort.124 When explaining their results, the authors found that the most predictive features were percentage of ventricular pacing and level of daytime activity. This use of AI to analyse near continuous ICD data has stimulated much interest and further studies.125 In an intriguing study by Sammani et al., deep neural networks were used to develop an autoencoder to represent key features of 1 million ECGs in a latent space; when applied to 695 patients with dilated cardiomyopathy, this interpretable AI predicted long-term VT/VF and found that P wave features, right bundle branch delay and reduced QRS-T voltages were the most predictive.126 Several studies applied AI to imaging data. Balaban et al. reported that remodelling of LV end-diastolic shape in 156 patients was the strongest multivariate predictor of VT/VF over an extended follow-up of 7.7 years.127
A remarkable achievement of AI has been to dramatically alter clinical care using simple data. Pioneering work by Attia et al. showed that the ‘AI-enabled 12-lead ECG’ in sinus rhythm can reveal left ventricular dysfunction128 and patients with paroxysmal AF.129 This is an exciting field, although further studies are needed since some others suggest that the AI-ECG may not add to traditional risk factors,130 or may not apply to single ECG leads76 in ambulatory monitors. AI may effectively ‘learn’ other ECG waveform patterns, for example AI of T-wave morphology was reported to identify gene-positive long QT syndrome patients from controls with a c-statistic of 0.901, better than QTc estimates.131 Convolutional neural networks applied to the ECG were shown to identify echocardiographic LV hypertrophy better than clinicians in 21 286 patients, with a c-statistic of 0.868 in an external validation set.132
The ESC-EHRA AF ablation long-term registry recently used AI of multimodal data to predict outcomes after AF ablation in 3128 patients with a c-statistic of 0.72, making the tool available online and outperforming clinical risk scores.133 AI has been applied to electronic health records to reduce spurious AF alerts, using natural language processing and CHA2DS2-VASc elements, providing 98% accuracy and reducing workload by 84%.134 AI of clinical data predicted sinus rhythm after electrical cardioversion of AF,135 and after guideline-directed medical therapy136 in secondary analyses of the Flec-SL-AFNET 3 and ANTIPAF-AFNET 2 trials, respectively. Neural network classifiers can predict recurrent syncope from patients in the emergency room using the history and ECG with accuracies from 67 to 95%.137
AI has been used to improve body surface potential mapping,138 and even to generate 3D maps of ventricular activation from the 12-lead ECG.139 AI of the ECG can separate typical from atypical atrial flutter mechanisms.140 A consensus document discussed the use of AI to better understand and map AF.70 Bhatia et al. applied AI to intracardiac electrograms in AF to identify patterns of organization associated with recurrence after ablation,141,142 and such tools have been incorporated into clinical mapping systems.143,144 Corrado et al recently applied AI to reveal tissue conduction slowing and atrial surface area that may predispose to re-entry during AF.145 Toprak reported that AI of NT-pro BNP and other circulating biomarkers improved upon traditional clinical variables in predicting incident AF.146
Europace has also taken the lead in reporting some of the challenges for AI. A notable editorial by Loring and Piccini in 2019 entitled ‘Machine Learning in Big Data: Handle with care’147 discussed how AI is not immune to bias in study design. These authors also showed that AI did not improve AF outcome predictions in the large ORBIT-AF and GARFIELD registries over traditional statistical predictors.148
In summary, AI is an extremely promising discipline to better understand and treat patients with heart rhythm disorders, and future work should focus on defining disease states, patient groups, and algorithmic approaches which will enable the greatest benefit. However, it is vital that the regulatory process is in balance with the development of novel models.
A regulatory perspective
Although our journey through digital arrhythmia care over the past decades has shown remarkable progress, there is also a need to be careful when introducing novel technologies. Medical devices are becoming smarter by using software that is increasingly ‘intelligent’, taking advantage of the steep rise in capabilities of AI and ML. As data is the cornerstone of AI learning, testing, and validation, this means that such novel devices need to comply (already or in the near future) with several regulations from the EU: besides the General Data Protection Regulation (GDPR) also the Medical Device Regulation (MDR), Data Governance Act, and the upcoming AI Act, and the European Health Data Space regulation. While this is already a significant challenge for small and even large manufacturing companies, for non-profit hospitals, and academic institutions it has become a major hurdle for the implementation of their innovations. Politicians and regulators across Europe have become aware of this issue, which is pushing innovation to other markets like the USA and China. Finding the proper balance between safety and innovation is still ongoing.94,149,150
One must weigh in that zero risk does not exist, and one always must consider the balance between benefit and risk for the individual patient and for society. Presently the balance seems to have swung towards risk aversity, which inadvertently creates risks of with-holding potentially beneficial devices from patients in need of them.151,152 There is also a disconnect between the regulatory requirements from GDPR and MDR and the scientific evidence on which clinicians base their decision to use certain devices in specific circumstances for a given patient. Scientific guidelines and the clinical requirements from GDPR and MDR aim towards the same goals at the highest level, but in their practical implementation, they do not coincide and sometimes only marginally overlap. The regulatory requirements focus on avoiding risk (which is further enhanced by the status of the notified bodies) and are subject to a variable interpretation of the GDPR in the EU Member States and of the MDR by the notified bodies. Scientific guidelines focus more on the benefit-risk balance but are not available for all clinical decisions and are often based on inconclusive or incomplete evidence and only on expert opinion with the inevitable (but mostly not intentional) bias.153
To bring the two requirement systems closer together and to avoid the high costs of duplicated clinical trials, registries, and studies, one could consider aligning them to their intrinsic purpose, which is allowing the patient/family to make the decisions about diagnostic and treatment options together with their health care provider based on the best available information, for example about benefits, risks, alternatives, and refraining from active therapy. Depending on the clinical situation of the patient, the severity of the pathology, and potential benefit of the intervention a lower or higher risk or uncertainty might be acceptable. It is the core of co-decision making to weigh these factors and come to an informed, balanced conclusion. The information needed to make these choices can vary and that must be reflected in the regulatory framework.
Similarly, the evidence to be provided by a manufacturer before the release of a product into the market should be based on the risk-benefit balance with a larger or smaller emphasis on post-release requirements. Regulators are presently hesitant to allow this because post-release obligations are often more difficult to define and enforce. For medical device software, this might possibly be the only way to address the difficulties with (self-)learning AI software and with the drift in the use of such devices in clinical real life.
In conclusion, the reality of medical device software, with its variable possibility of extensive pre-release clinical testing and potential drift in use and impact, will necessitate a more balanced risk-benefit evaluation and alignment of the regulatory and clinical scientific standards in the future.
The future digital aspects for electrophysiology Europace
As described in the previous sections, EP has a history of utilizing advanced digital solutions and tools. With the current rapid advancement of digital technologies, commonly referred to as digital transformation, including AI using ML and deep learning, RM, wearables, and advanced imaging, we can expect even greater progress in the field. Some potential future aspects for digital in cardiovascular EP follow.
Telemedicine and RM, as discussed in the section on virtual clinics and telemonitoring, have become increasingly important topics in cardiology,75 especially with the acceleration brought on by the COVID-19 pandemic. We could expect to see improved capabilities and infrastructures for RM,68 as well as advancements in wearable technologies. With ongoing developments in device miniaturization and wearable tech, monitoring, and treatment options should become more convenient and patient friendly. For instance, implantable sensors or wearable patches may provide continuous monitoring of heart rhythm and other relevant data, enabling early detection and intervention in case of abnormalities. This should lead to better patient outcomes and satisfaction, as well as a reduced workload for healthcare staff and lower healthcare costs.
ML and AI algorithms have the potential to transform EP by enabling automated and precise analysis of vast amounts of data.63,154,155 These technologies can aid in predicting, diagnosing, and providing personalized treatment for heart rhythm disorders. The rapid development of digital technologies is expected to lead to significant improvements in imaging and mapping techniques, resulting in better visualization and characterization of the heart’s electrical activity. This could include the widespread adoption of high-resolution imaging and three-dimensional mapping technologies, which would enable more precise diagnosis and treatment planning for patients. In addition, ML-powered algorithms can optimize the outcomes of catheter ablations by providing real-time guidance and feedback.
Advanced imaging using virtual reality and augmented reality has the potential to revolutionize EP training and procedural planning. Virtual reality can provide a safe and controlled environment for simulating ablation procedures, reducing the learning curve and improving safety. Additionally, augmented reality can offer real-time visual guidance during procedures by overlaying relevant information, such as anatomical landmarks or electrode placement, onto the patient’s body. These technologies could ultimately lead to better patient outcomes. Moreover, virtual reality can also be utilized to reduce patient anxiety by aiding in teaching and preparing patients for procedures, as well as assisting in post-procedural rehabilitation.156
One of the most challenging yet promising areas where AI could have a significant impact is personalized medicine. AI-based algorithms that incorporate individual patient characteristics, such as genetics, lifestyle, and medical history, can provide advanced analytics and computational modelling to predict the optimal treatment approach for each patient. This approach can lead to more targeted and effective treatments with improved outcomes.157
In conclusion, the future of cardiovascular EP will be shaped by rapid advancements in digital technologies, such as advanced imaging and mapping, AI and ML, telemedicine and RM, virtual and augmented reality, miniaturization and wearable devices, and personalized medicine. These advancements have the potential to significantly improve diagnosis, treatment, and patient outcomes in cardiovascular EP, and represent an exciting time for the field. With this reflection on the past quarter of a century in EP, we can now cast our eyes forward, envisioning that the journey ahead will likely accelerate our digital knowledge. In the coming 25 years in the EP Europace journal, we will continue to provide you with novel digital tools to improve the management of arrhythmia patients and steadfastly aim to increase our coverage of digital topics, using a scientific approach to enable better patient management.
Contributor Information
Emma Svennberg, Department of Medicine Huddinge, Karolinska Institutet, Karolinska University Hospital Huddinge, SE-141 86 Stockholm, Sweden.
Enrico G Caiani, Politecnico di Milano, Electronic, Information and Biomedical Engineering Department, Milan, Italy; Istituto Auxologico Italiano IRCCS, Milan, Italy.
Nico Bruining, Department of Clinical and Experimental Information processing (Digital Cardiology), Erasmus Medical Center, Thoraxcenter, Rotterdam, The Netherlands.
Lien Desteghe, Research Group Cardiovascular Diseases, University of Antwerp, 2000 Antwerp, Belgium; Department of Cardiology, Antwerp University Hospital, 2056 Edegem, Belgium; Faculty of Medicine and Life Sciences, Hasselt University, 3500 Hasselt, Belgium; Department of Cardiology, Heart Centre Hasselt, Jessa Hospital, 3500 Hasselt, Belgium.
Janet K Han, Division of Cardiology, Veterans Affairs Greater Los Angeles Healthcare System, Los Angeles, CA 90073, USA; Cardiac Arrhythmia Center, University of California Los Angeles, Los Angeles, CA, USA.
Sanjiv M Narayan, Cardiology Division, Cardiovascular Institute and Institute for Computational and Mathematical Engineering, Stanford University, Stanford, CA, USA.
Frank E Rademakers, Department of Cardiology, KU Leuven, 3000 Leuven, Belgium.
Prashanthan Sanders, Centre for Heart Rhythm Disorders, University of Adelaide and Royal Adelaide Hospital, 5005 Adelaide, Australia.
David Duncker, Hannover Heart Rhythm Center, Department of Cardiology and Angiology, Hannover Medical School, Hannover, Germany.
Funding
ES is supported by a Grant from Region Stockholm, the Swedish Heart and Lung foundation, CIMED and Swedish Research Council Vetenskaps Rådet DNR (2022-01466) PS is supported by an Investigator Grant from the National Health and Medical Research Council of Australia.
References
- 1. Halimi F, Clémenty J, Attuel P, Dessenne X, Amara W. Optimized post-operative surveillance of permanent pacemakers by home monitoring: the OEDIPE trial. Europace 2008;10:1392–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Heidbüchel H, Lioen P, Foulon S, Huybrechts W, Ector J, Willems Ret al. . Potential role of remote monitoring for scheduled and unscheduled evaluations of patients with an implantable defibrillator. Europace 2008;10:351–7. [DOI] [PubMed] [Google Scholar]
- 3. Neuzil P, Taborsky M, Holy F, Wallbrueck K. Early automatic remote detection of combined lead insulation defect and ICD damage. Europace 2008;10:556–7. [DOI] [PubMed] [Google Scholar]
- 4. Nielsen JC, Kottkamp H, Zabel M, Aliot E, Kreutzer U, Bauer Aet al. . Automatic home monitoring of implantable cardioverter defibrillators. Europace 2008;10:729–35. [DOI] [PubMed] [Google Scholar]
- 5. Perings C, Klein G, Toft E, Moro C, Klug D, Böcker Det al. . The RIONI study rationale and design: validation of the first stored electrograms transmitted via home monitoring in patients with implantable defibrillators. Europace 2006;8:288–92. [DOI] [PubMed] [Google Scholar]
- 6. Raatikainen MJ, Uusimaa P, van Ginneken MM, Janssen JP, Linnaluoto M. Remote monitoring of implantable cardioverter defibrillator patients: a safe, time-saving, and cost-effective means for follow-up. Europace 2008;10:1145–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dubner S, Auricchio A, Steinberg JS, Vardas P, Stone P, Brugada Jet al. . ISHNE/EHRA expert consensus on remote monitoring of cardiovascular implantable electronic devices (CIEDs). Europace 2012;14:278–93. [DOI] [PubMed] [Google Scholar]
- 8. Simovic S, Providencia R, Barra S, Kircanski B, Guerra JM, Conte Get al. . The use of remote monitoring of cardiac implantable devices during the COVID-19 pandemic: an EHRA physician survey. Europace 2022;24:473–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Crossley GH, Chen J, Choucair W, Cohen TJ, Gohn DC, Johnson WBet al. . Clinical benefits of remote versus transtelephonic monitoring of implanted pacemakers. J Am Coll Cardiol 2009;54:2012–9. [DOI] [PubMed] [Google Scholar]
- 10. Al-Khatib SM, Piccini JP, Knight D, Stewart M, Clapp-Channing N, Sanders GD. Remote monitoring of implantable cardioverter defibrillators versus quarterly device interrogations in clinic: results from a randomized pilot clinical trial. J Cardiovasc Electrophysiol 2010;21:545–50. [DOI] [PubMed] [Google Scholar]
- 11. Russo V, Rapacciuolo A, Rago A, Tavoletta V, De Vivo S, Ammirati Get al. . Early evaluation of atrial high rate episodes using remote monitoring in pacemaker patients: results from the RAPID study. J Arrhythm 2022;38:213–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Crossley GH, Boyle A, Vitense H, Chang Y, Mead RH. The CONNECT (clinical evaluation of remote notification to reduce time to clinical decision) trial: the value of wireless remote monitoring with automatic clinician alerts. J Am Coll Cardiol 2011;57:1181–9. [DOI] [PubMed] [Google Scholar]
- 13. Burri H, Senouf D. Remote monitoring and follow-up of pacemakers and implantable cardioverter defibrillators. Europace 2009;11:701–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ricci RP, Morichelli L, Santini M. Remote control of implanted devices through home monitoring technology improves detection and clinical management of atrial fibrillation. Europace 2009;11:54–61. [DOI] [PubMed] [Google Scholar]
- 15. Perings C, Bauer WR, Bondke HJ, Mewis C, James M, Bocker Det al. . Remote monitoring of implantable-cardioverter defibrillators: results from the reliability of IEGM online interpretation (RIONI) study. Europace 2011;13:221–9. [DOI] [PubMed] [Google Scholar]
- 16. Varma N, Epstein AE, Irimpen A, Schweikert R, Love C. Efficacy and safety of automatic remote monitoring for implantable cardioverter-defibrillator follow-up: the lumos-T safely reduces routine office device follow-up (TRUST) trial. Circulation 2010;122:325–32. [DOI] [PubMed] [Google Scholar]
- 17. Varma N, Pavri BB, Stambler B, Michalski J. Same-day discovery of implantable cardioverter defibrillator dysfunction in the TRUST remote monitoring trial: influence of contrasting messaging systems. Europace 2013;15:697–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Landolina M, Perego GB, Lunati M, Curnis A, Guenzati G, Vicentini Aet al. . Remote monitoring reduces healthcare use and improves quality of care in heart failure patients with implantable defibrillators: the evolution of management strategies of heart failure patients with implantable defibrillators (EVOLVO) study. Circulation 2012;125:2985–92. [DOI] [PubMed] [Google Scholar]
- 19. Mabo P, Victor F, Bazin P, Ahres S, Babuty D, Da Costa Aet al. . A randomized trial of long-term remote monitoring of pacemaker recipients (the COMPAS trial). Eur Heart J 2012;33:1105–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hindricks G, Elsner C, Piorkowski C, Taborsky M, Geller JC, Schumacher Bet al. . Quarterly vs. Yearly clinical follow-up of remotely monitored recipients of prophylactic implantable cardioverter-defibrillators: results of the REFORM trial. Eur Heart J 2014;35:98–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Guédon-Moreau L, Lacroix D, Sadoul N, Clémenty J, Kouakam C, Hermida JSet al. . Costs of remote monitoring vs. ambulatory follow-ups of implanted cardioverter defibrillators in the randomized ECOST study. Europace 2014;16:1181–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zanaboni P, Landolina M, Marzegalli M, Lunati M, Perego GB, Guenzati Get al. . Cost-utility analysis of the EVOLVO study on remote monitoring for heart failure patients with implantable defibrillators: randomized controlled trial. J Med Internet Res 2013;15:e106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Becher J, Kaufmann SG, Paule S, Fahn B, Skerl O, Bauer WRet al. . Device-based impedance measurement is a useful and accurate tool for direct assessment of intrathoracic fluid accumulation in heart failure. Europace 2010;12:731–40. [DOI] [PubMed] [Google Scholar]
- 24. Chew DS, Zarrabi M, You I, Morton J, Low A, Reyes Let al. . Clinical and economic outcomes associated with remote monitoring for cardiac implantable electronic devices: A population-based analysis. Can J Cardiol 2022;38:736–44. [DOI] [PubMed] [Google Scholar]
- 25. Burri H, Heidbüchel H, Jung W, Brugada P. Remote monitoring: a cost or an investment? Europace 2011;13:ii44–8. [DOI] [PubMed] [Google Scholar]
- 26. Burri H, Sticherling C, Wright D, Makino K, Smala A, Tilden D. Cost-consequence analysis of daily continuous remote monitoring of implantable cardiac defibrillator and resynchronization devices in the UK. Europace 2013;15:1601–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chronaki CE, Vardas P. Remote monitoring costs, benefits, and reimbursement: a European perspective. Europace 2013;15:i59–64. [DOI] [PubMed] [Google Scholar]
- 28. Ricci RP, D'Onofrio A, Padeletti L, Sagone A, Vicentini A, Vincenti Aet al. . Rationale and design of the health economics evaluation registry for remote follow-up: TARIFF. Europace 2012;14:1661–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sequeira S, Jarvis CI, Benchouche A, Seymour J, Tadmouri A. Cost-effectiveness of remote monitoring of implantable cardioverter-defibrillators in France: a meta-analysis and an integrated economic model derived from randomized controlled trials. Europace 2020;22:1071–82. [DOI] [PubMed] [Google Scholar]
- 30. Slotwiner D, Wilkoff B. Cost efficiency and reimbursement of remote monitoring: a US perspective. Europace 2013;15:i54–8. [DOI] [PubMed] [Google Scholar]
- 31. Hindricks G, Varma N, Kacet S, Lewalter T, Søgaard P, Guédon-Moreau Let al. . Daily remote monitoring of implantable cardioverter-defibrillators: insights from the pooled patient-level data from three randomized controlled trials (IN-TIME, ECOST, TRUST). Eur Heart J 2017;38:1749–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Parthiban N, Esterman A, Mahajan R, Twomey DJ, Pathak RK, Lau DHet al. . Remote monitoring of implantable cardioverter-defibrillators: A systematic review and meta-analysis of clinical outcomes. J Am Coll Cardiol 2015;65:2591–600. [DOI] [PubMed] [Google Scholar]
- 33. Varma N, Piccini JP, Snell J, Fischer A, Dalal N, Mittal Set al. . The relationship between level of adherence to automatic wireless remote monitoring and survival in pacemaker and defibrillator patients. J Am Coll Cardiol 2015;65:2601–10. [DOI] [PubMed] [Google Scholar]
- 34. Kurek A, Tajstra M, Gadula-Gacek E, Buchta P, Skrzypek M, Pyka Let al. . Impact of remote monitoring on long-term prognosis in heart failure patients in a real-world cohort: results from all-comers COMMIT-HF trial. J Cardiovasc Electrophysiol 2017;28:425–31. [DOI] [PubMed] [Google Scholar]
- 35. Saxon LA, Hayes DL, Gilliam FR, Heidenreich PA, Day J, Seth Met al. . Long-term outcome after ICD and CRT implantation and influence of remote device follow-up: the ALTITUDE survival study. Circulation 2010;122:2359–67. [DOI] [PubMed] [Google Scholar]
- 36. Hernández-Madrid A, Lewalter T, Proclemer A, Pison L, Lip GYH, Blomstrom-Lundqvist C. Remote monitoring of cardiac implantable electronic devices in Europe: results of the European heart rhythm association survey. Europace 2014;16:129–32. [DOI] [PubMed] [Google Scholar]
- 37. Gillis AM. Expert commentary: how well has the call from Heart Rhythm Society/European Heart Rhythm Association for improved device monitoring been answered? Europace 2013;15:i32–4. [DOI] [PubMed] [Google Scholar]
- 38. Rosenfeld LE, Patel AS, Ajmani VB, Holbrook RW, Brand TA. Compliance with remote monitoring of ICDS/CRTDS in a real-world population. Pacing Clin Electrophysiol 2014;37:820–7. [DOI] [PubMed] [Google Scholar]
- 39. O'Shea CJ, Middeldorp ME, Hendriks JM, Brooks AG, Lau DH, Emami Met al. . Remote monitoring alert burden: an analysis of transmission in >26,000 patients. JACC Clin Electrophysiol 2021;7:226–34. [DOI] [PubMed] [Google Scholar]
- 40. Afzal MR, Mease J, Koppert T, Okabe T, Tyler J, Houmsse Met al. . Incidence of false-positive transmissions during remote rhythm monitoring with implantable loop recorders. Heart Rhythm 2020;17:75–80. [DOI] [PubMed] [Google Scholar]
- 41. O'Shea CJ, Middeldorp ME, Hendriks JM, Brooks AG, Harper C, Thomas Get al. . Remote monitoring of implantable loop recorders: false-positive alert episode burden. Circ Arrhythm Electrophysiol 2021;14:e009635. [DOI] [PubMed] [Google Scholar]
- 42. Mittal S, Oliveros S, Li J, Barroyer T, Henry C, Gardella C. AI Filter improves positive predictive value of atrial fibrillation detection by an implantable loop recorder. JACC Clin Electrophysiol 2021;7:965–75. [DOI] [PubMed] [Google Scholar]
- 43. O'Shea CJ, Brooks AG, Middeldorp ME, Harper C, Hendriks JM, Russo AMet al. . Device-detected atrial fibrillation in a large remote-monitored cohort: implications for anticoagulation and need for new pathways of service delivery. J Interv Card Electrophysiol 2023. (EPUB ahead of print: 2023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ferrick AM, Raj SR, Deneke T, Kojodjojo P, Lopez-Cabanillas N, Abe Het al. . 2023 HRS/EHRA/APHRS/LAHRS expert consensus statement on practical management of the remote device clinic. Europace 2023;25:euad123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Boriani G, Burri H, Svennberg E, Imberti JF, Merino JL, Leclercq Cet al. . Current status of reimbursement practices for remote monitoring of cardiac implantable electrical devices across Europe. Europace 2022;24:1875–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Carpenter A, Frontera A. Smart-watches: a potential challenger to the implantable loop recorder? Europace 2016;18:791–3. [DOI] [PubMed] [Google Scholar]
- 47. Vardas P, Cowie M, Dagres N, Asvestas D, Tzeis S, Vardas EPet al. . The electrocardiogram endeavour: from the Holter single-lead recordings to multilead wearable devices supported by computational machine learning algorithms. Europace 2020;22:19–23. [DOI] [PubMed] [Google Scholar]
- 48. Enseleit F, Duru F. Long-term continuous external electrocardiographic recording: a review. Europace 2006;8:255–66. [DOI] [PubMed] [Google Scholar]
- 49. Kamalvand K, Tan K, Kotsakis A, Bucknall C, Sulke N. Ambulatory patient-activated arrhythmia monitoring: comparison of a new wrist-applied monitor with a conventional precordial device. J Electrocardiol 1997;30:127–31. [DOI] [PubMed] [Google Scholar]
- 50. Di Rienzo M, Racca V, Rizzo F, Bordoni B, Parati G, Castiglioni Pet al. . Evaluation of a textile-based wearable system for the electrocardiogram monitoring in cardiac patients. Europace 2013;15:607–12. [DOI] [PubMed] [Google Scholar]
- 51. Svennberg E, Tjong F, Goette A, Akoum N, Di Biase L, Bordachar Pet al. . How to use digital devices to detect and manage arrhythmias: an EHRA practical guide. Europace 2022;24:979–1005. [DOI] [PubMed] [Google Scholar]
- 52. Hermans ANL, van der Velden RMJ, Gawalko M, Verhaert DVM, Desteghe L, Duncker Det al. . On-demand mobile health infrastructures to allow comprehensive remote atrial fibrillation and risk factor management through teleconsultation. Clin Cardiol 2020;43:1232–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Väliaho ES, Kuoppa P, Lipponen JA, Martikainen TJ, Jäntti H, Rissanen TTet al. . Wrist band photoplethysmography in detection of individual pulses in atrial fibrillation and algorithm-based detection of atrial fibrillation. Europace 2019;21:1031–8. [DOI] [PubMed] [Google Scholar]
- 54. Healey JS, Wong J. Wearable and implantable diagnostic monitors in early assessment of atrial tachyarrhythmia burden. Europace 2019;21:377–82. [DOI] [PubMed] [Google Scholar]
- 55. Sandberg EL, Halvorsen S, Berge T, Grimsmo J, Atar D, Fensli Ret al. . Fully digital self-screening for atrial fibrillation with patch electrocardiogram. Europace 2023;25:euad075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Roger A, Cottin Y, Bentounes SA, Bisson A, Bodin A, Herbert Jet al. . Incidence of clinical atrial fibrillation and related complications using a screening algorithm at a nationwide level. Europace 2023;25:euad063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Perez MV, Mahaffey KW, Hedlin H, Rumsfeld JS, Garcia A, Ferris Tet al. . Large-Scale assessment of a smartwatch to identify atrial fibrillation. N Engl J Med 2019;381:1909–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Guo Y, Wang H, Zhang H, Liu T, Liang Z, Xia Yet al. . Mobile photoplethysmographic technology to detect atrial fibrillation. J Am Coll Cardiol 2019;74:2365–75. [DOI] [PubMed] [Google Scholar]
- 59. Lubitz SA, Faranesh AZ, Selvaggi C, Atlas SJ, McManus DD, Singer DEet al. . Detection of atrial fibrillation in a large population using wearable devices: the fitbit heart study. Circulation 2022;146:1415–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Svennberg E, Friberg L, Frykman V, Al-Khalili F, Engdahl J, Rosenqvist M. Clinical outcomes in systematic screening for atrial fibrillation (STROKESTOP): a multicentre, parallel group, unmasked, randomised controlled trial. Lancet 2021;398:1498–506. [DOI] [PubMed] [Google Scholar]
- 61. Lyth J, Svennberg E, Bernfort L, Aronsson M, Frykman V, Al-Khalili Fet al. . Cost-effectiveness of population screening for atrial fibrillation: the STROKESTOP study. Eur Heart J 2023;44:196–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist Cet al. . 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J 2021;42:373–498. [DOI] [PubMed] [Google Scholar]
- 63. Schnabel RB, Marinelli EA, Arbelo E, Boriani G, Boveda S, Buckley CMet al. . Early diagnosis and better rhythm management to improve outcomes in patients with atrial fibrillation: the 8th AFNET/EHRA consensus conference. Europace 2023;25:6–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Manninger M, Kosiuk J, Zweiker D, Njeim M, Antolic B, Kircanski Bet al. . Role of wearable rhythm recordings in clinical decision making-ThewEHRAblesproject. Clin Cardiol 2020;43:1032–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Manninger M, Zweiker D, Svennberg E, Chatzikyriakou S, Pavlovic N, Zaman JABet al. . Current perspectives on wearable rhythm recordings for clinical decision-making: the wEHRAbles 2 survey. Europace 2021;23:1106–13. [DOI] [PubMed] [Google Scholar]
- 66. Ding EY, Svennberg E, Wurster C, Duncker D, Manninger M, Lubitz SAet al. . Survey of current perspectives on consumer-available digital health devices for detecting atrial fibrillation. Cardiovasc Digit Health J 2020;1:21–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Gawałko M, Duncker D, Manninger M, van der Velden RMJ, Hermans ANL, Verhaert DVMet al. . The European TeleCheck-AF project on remote app-based management of atrial fibrillation during the COVID-19 pandemic: centre and patient experiences. Europace 2021;23:1003–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Pluymaekers N, Hermans ANL, van der Velden RMJ, Gawałko M, den Uijl DW, Buskes Set al. . Implementation of an on-demand app-based heart rate and rhythm monitoring infrastructure for the management of atrial fibrillation through teleconsultation: TeleCheck-AF. Europace 2021;23:345–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Linz D, Hermans A, Tieleman RG. Early atrial fibrillation detection and the transition to comprehensive management. Europace 2021;23:ii46–51. [DOI] [PubMed] [Google Scholar]
- 70. de Groot NMS, Shah D, Boyle PM, Anter E, Clifford GD, Deisenhofer Iet al. . Critical appraisal of technologies to assess electrical activity during atrial fibrillation: a position paper from the European Heart Rhythm Association and European Society of Cardiology Working Group on eCardiology in collaboration with the Heart Rhythm Society, Asia Pacific Heart Rhythm Society, Latin American Heart Rhythm Society and Computing in Cardiology. Europace 2022;24:313–30. [DOI] [PubMed] [Google Scholar]
- 71. Lopez Perales CR, Van Spall HGC, Maeda S, Jimenez A, Laţcu DG, Milman Aet al. . Mobile health applications for the detection of atrial fibrillation: a systematic review. Europace 2021;23:11–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. van der Velden RMJ, Verhaert DVM, Hermans ANL, Duncker D, Manninger M, Betz Ket al. . The photoplethysmography dictionary: practical guidance on signal interpretation and clinical scenarios from TeleCheck-AF. Eur Heart J Digital Health 2021;2:363–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Gruwez H, Evens S, Proesmans T, Duncker D, Linz D, Heidbuchel Het al. . Accuracy of physicians interpreting photoplethysmography and electrocardiography tracings to detect atrial fibrillation: INTERPRET-AF. Front Cardiovasc Med 2021;8:734737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Boriani G, Svennberg E, Guerra F, Linz D, Casado-Arroyo R, Malaczynska-Rajpold Ket al. . Reimbursement practices for use of digital devices in atrial fibrillation and other arrhythmias: a European Heart Rhythm Association survey. Europace 2022;24:1834–43. [DOI] [PubMed] [Google Scholar]
- 75. Leclercq C, Witt H, Hindricks G, Katra RP, Albert D, Belliger Aet al. . Wearables, telemedicine, and artificial intelligence in arrhythmias and heart failure: proceedings of the European Society of Cardiology Cardiovascular Round Table. Europace 2022;24:1372–83. [DOI] [PubMed] [Google Scholar]
- 76. Hygrell T, Viberg F, Dahlberg E, Charlton PH, Kemp Gudmundsdottir K, Mant Jet al. . An artificial intelligence-based model for prediction of atrial fibrillation from single-lead sinus rhythm electrocardiograms facilitating screening. Europace 2023;25:1332–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Hermans ANL, Gawałko M, Hillmann HAK, Sohaib A, van der Velden RMJ, Betz Ket al. . Self-Reported Mobile health-based risk factor and CHA(2)DS(2)-VASc-score assessment in patients with atrial fibrillation: TeleCheck-AF results. Front Cardiovasc Med 2021;8:757587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Gawałko M, Hermans AN, van der Velden RM, Betz K, Vm Verhaert D, Hillmann HAKet al. . Patient motivation and adherence to an on-demand app-based heart rate and rhythm monitoring for atrial fibrillation management: data from the TeleCheck-AF project. Eur J Cardiovasc Nurs 2023;22:412–24. [DOI] [PubMed] [Google Scholar]
- 79. GSMA Association, The Mobile Economy Europe 2022, accessed 15 May 2023, https://www.gsma.com/mobileeconomy/wp-content/uploads/2022/10/051022-Mobile-Economy-Europe-2022.pdf.
- 80. Burri H, Mondouagne Engkolo LP, Dayal N, Etemadi A, Makhlouf AM, Stettler Cet al. . Low risk of electromagnetic interference between smartphones and contemporary implantable cardioverter defibrillators. Europace 2016;18:726–31. [DOI] [PubMed] [Google Scholar]
- 81. Desteghe L, Raymaekers Z, Lutin M, Vijgen J, Dilling-Boer D, Koopman Pet al. . Performance of handheld electrocardiogram devices to detect atrial fibrillation in a cardiology and geriatric ward setting. Europace 2017;19:29–39. [DOI] [PubMed] [Google Scholar]
- 82. Kotecha D, Kirchhof P. ESC apps for atrial fibrillation. Eur Heart J 2017;38:2643–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Kotecha D, Chua WWL, Fabritz L, Hendriks J, Casadei B, Schotten Uet al. . European Society of Cardiology smartphone and tablet applications for patients with atrial fibrillation and their health care providers. Europace 2018;20:225–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Coorey GM, Neubeck L, Mulley J, Redfern J. Effectiveness, acceptability and usefulness of mobile applications for cardiovascular disease self-management: systematic review with meta-synthesis of quantitative and qualitative data. Eur J Prev Cardiol 2018;25:505–21. [DOI] [PubMed] [Google Scholar]
- 85. Gandhi S, Chen S, Hong L, Sun K, Gong E, Li Cet al. . Effect of Mobile health interventions on the secondary prevention of cardiovascular disease: systematic review and meta-analysis. Can J Cardiol 2017;33:219–31. [DOI] [PubMed] [Google Scholar]
- 86. Pfaeffli Dale L, Dobson R, Whittaker R, Maddison R. The effectiveness of mobile-health behaviour change interventions for cardiovascular disease self-management: A systematic review. Eur J Prev Cardiol 2016;23:801–17. [DOI] [PubMed] [Google Scholar]
- 87. Lane DA, McMahon N, Gibson J, Weldon JC, Farkowski MM, Lenarczyk Ret al. . Mobile health applications for managing atrial fibrillation for healthcare professionals and patients: a systematic review. Europace 2020;22:1567–78. [DOI] [PubMed] [Google Scholar]
- 88. Indraratna P, Tardo D, Yu J, Delbaere K, Brodie M, Lovell Net al. . Mhealth interventions in the management of heart failure, ischaemic heart disease and hypertension: a systematic review and meta-analysis of randomised controlled trials. Euro Heart Jl 2020;41:ehaa946.3507. [Google Scholar]
- 89. Linz D, Pluymaekers N, Duncker D, Hermans ANL, Verhaert DVM, Pison Let al. . The TeleCheck-AF project on remote app-based management of atrial fibrillation during the COVID-19 pandemic: patient experiences. Europace 2021;23:1003–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Hermans A, Gawalko M, Pluymaekers N, Verhaert DVM, Van Der Velden RMJ, Betz Ket al. . Mobile app-based symptom-rhythm correlation assessment in patients with persistent atrial fibrillation. Europace 2022;24:euac053.577. [DOI] [PubMed] [Google Scholar]
- 91. Gawalko M, Hermans A, Van Der Velden R, Betz K, Verhaert DVM, Pluymaekers NAHAet al. . Patient motivation and adherence to an on-demand app-based heart rate and rhythm monitoring infrastructure for atrial fibrillation management through teleconsultation. TeleCheck-AF project results. Europace 2022;24:euac053.587. [Google Scholar]
- 92. Manyam H, Burri H, Casado-Arroyo R, Varma N, Lennerz C, Klug Det al. . Smartphone-based cardiac implantable electronic device remote monitoring: improved compliance and connectivity. Eur Heart J Digital Health 2022;4:43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Varma N, Cygankiewicz I, Turakhia M, Heidbuchel H, Hu Y, Chen LYet al. . 2021 ISHNE/HRS/EHRA/APHRS collaborative statement on mHealth in arrhythmia management: digital medical tools for heart rhythm professionals: from the International Society for Holter and Noninvasive Electrocardiology/Heart Rhythm Society/European Heart Rhythm Association/Asia Pacific Heart Rhythm Society. Eur Heart J Digital Health 2021;2:7–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Nielsen JC, Kautzner J, Casado-Arroyo R, Burri H, Callens S, Cowie MRet al. . Remote monitoring of cardiac implanted electronic devices: legal requirements and ethical principles—ESC Regulatory Affairs Committee/EHRA joint task force report. Europace 2020;22:1742–58. [DOI] [PubMed] [Google Scholar]
- 95. Guo Y, Chen Y, Lane DA, Liu L, Wang Y, Lip GYH. Mobile health technology for atrial fibrillation management integrating decision support, education, and patient involvement: mAF app trial. Am J Med 2017;130:1388–96.e6. [DOI] [PubMed] [Google Scholar]
- 96. Knaepen L, Delesie M, Theunis R, Vijgen J, Dendale P, Desteghe Let al. . A new smartphone application for integrated transmural care of atrial fibrillation. AF-EduApp: usability and validation study. Digit Health 2021;7:20552076211067105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Bruining N, Caiani E, Chronaki C, Guzik P, van der Velde E. Acquisition and analysis of cardiovascular signals on smartphones: potential, pitfalls and perspectives: by the Task Force of the e-Cardiology Working Group of European Society of Cardiology. Eur J Prev Cardiol 2020;21:4–13. [DOI] [PubMed] [Google Scholar]
- 98. Frederix I, Caiani EG, Dendale P, Anker S, Bax J, Böhm Aet al. . ESC e-Cardiology Working Group Position Paper: overcoming challenges in digital health implementation in cardiovascular medicine. Eur J Prev Cardiol 2020;26:1166–77. [DOI] [PubMed] [Google Scholar]
- 99. Fraser AG, Byrne RA, Kautzner J, Butchart EG, Szymański P, Leggeri Iet al. . Implementing the new European regulations on medical devices-clinical responsibilities for evidence-based practice: a report from the Regulatory Affairs Committee of the European Society of Cardiology. Eur Heart J 2020;41:2589–96. [DOI] [PubMed] [Google Scholar]
- 100. Furman S, Parker B, Escher DJ. Transtelephone pacemaker clinic. J Thorac Cardiovasc Surg 1971;61:827–34. [PubMed] [Google Scholar]
- 101. Lakkireddy DR, Chung MK, Gopinathannair R, Patton KK, Gluckman TJ, Turagam Met al. . Guidance for cardiac electrophysiology during the COVID-19 pandemic from the Heart Rhythm Society COVID-19 Task Force; Electrophysiology Section of the American College of Cardiology; and the Electrocardiography and Arrhythmias Committee of the Council on Clinical Cardiology, American Heart Association. Heart Rhythm 2020;17:e233–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Varma N, Marrouche NF, Aguinaga L, Albert CM, Arbelo E, Choi JIet al. . HRS/EHRA/APHRS/LAHRS/ACC/AHA worldwide practice update for telehealth and arrhythmia monitoring during and after a pandemic. Europace 2021;23:313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Han JK, Al-Khatib SM, Albert CM. Changes in the digital health landscape in cardiac electrophysiology: A pre-and peri-pandemic COVID-19 era survey. Cardiovascr Digital Health J 2021;2:55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Kalwani NM, Osmanlliu E, Parameswaran V, Qureshi L, Dash R, Heidenreich PAet al. . Changes in telemedicine use and ambulatory visit volumes at a multispecialty cardiovascular center during the COVID-19 pandemic. J Telemed Telecare 2022:1357633X211073428. (EPUB ahead of print: 2022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Guo Y, Lane DA, Chen Y, Lip GYH. Mobile health technology facilitates population screening and integrated care management in patients with atrial fibrillation. Eur Heart J 2020;41:1617–9. [DOI] [PubMed] [Google Scholar]
- 106. Hu PT, Hilow H, Patel D, Eppich M, Cantillon D, Tchou Pet al. . Use of virtual visits for the care of the arrhythmia patient. Heart Rhythm 2020;17:1779–83. [DOI] [PubMed] [Google Scholar]
- 107. Shatla I, El-Zein RS, Ubaid A, ElBallat A, Sammour Y, Kennedy KFet al. . An analysis of telehealth in the outpatient management of atrial fibrillation during the COVID-19 pandemic. Am J Cardiol 2023;192:174–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Mariani MV, Pierucci N, Forleo GB, Schiavone M, Bernardini A, Gasperetti Aet al. . The feasibility, effectiveness and acceptance of virtual visits as compared to in-person visits among clinical electrophysiology patients during the COVID-19 pandemic. J Clin Med 2023;12:620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Zenger B, Jared Bunch T. How simple ideas forged in the fire of adversity can change healthcare: telehealth for atrial fibrillation during the COVID 19 pandemic. Europace 2021;23:1153–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Linz D, Pluymaekers N, Hendriks JM. TeleCheck-AF for COVID-19. Eur Heart J 2020;41:1954–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Kotb A, Armstrong S, Antoun I, Koev I, Mavilakandy A, Barker Jet al. . Atrial fibrillation virtual ward: reshaping the future of AF care. Eur Heart J 2022;43:ehac544.2804. [Google Scholar]
- 112. Waterman AD, Banet G, Milligan PE, Frazier A, Verzino E, Walton Bet al. . Patient and physician satisfaction with a telephone-based anticoagulation service. J Gen Intern Med 2001;16:460–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Wittkowsky AK, Nutescu EA, Blackburn J, Mullins J, Hardman J, Mitchell Jet al. . Outcomes of oral anticoagulant therapy managed by telephone vs in-office visits in an anticoagulation clinic setting. Chest 2006;130:1385–9. [DOI] [PubMed] [Google Scholar]
- 114. Shah RL, Kapoor R, Bonnett C, Ottoboni LK, Tacklind C, Tsiperfal Aet al. . Antiarrhythmic drug loading at home using remote monitoring: a virtual feasibility study during COVID-19 social distancing. Eur Heart J Digital Health 2021;2:259–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Labreck M, Badin A, Fu EY, Chopra N, Billakanty SR, Tyler Jet al. . Pharmacist driven outpatient sotalol loading protocol. Cardiovascu Digital Health J 2022;3:S5. [Google Scholar]
- 116. Manimaran M, Das D, Martinez P, Schwartz R, Schilling R, Finlay M. The impact of virtual arrhythmia clinics following catheter ablation for atrial fibrillation. Eur Heart J Qual Care Clin Outcomes 2019;5:272–3. [DOI] [PubMed] [Google Scholar]
- 117. Lambert CT, Patel D, Bumgarner JM, Kanj M, Cantillon D, Saliba Wet al. . Atrial fibrillation future clinic. Novel platform to integrate smart device electrocardiogram into clinical practice. Cardiovasc Digital Health J 2021;2:92–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Weng W, Blanchard C, Reed JL, Matheson K, McIntyre C, Gray Cet al. . A virtual platform to deliver ambulatory care for patients with atrial fibrillation. Cardiovasc Digit Health J 2021;2:63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Rush KL, Burton L, Loewen P, Wilson R, Singh S, Moroz Let al. . Patients’ experiences with the fit of virtual atrial fibrillation care during the pandemic: qualitative descriptive study. JMIR Cardio 2023;7:e41548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Krittanawong C, Johnson KW, Rosenson RS, Wang Z, Aydar M, Baber Uet al. . Deep learning for cardiovascular medicine: a practical primer. Eur Heart J 2019;40:2058–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Rojo-Alvarez JL, Arenal A, García-Alberola A, Ortiz M, Valdés M, Artés-Rodríguez A. A new algorithm for rhythm discrimination in cardioverter defibrillators based on the initial voltage changes of the ventricular electrogram. Europace 2003;5:77–82. [DOI] [PubMed] [Google Scholar]
- 122. Theuns DA, Rivero-Ayerza M, Goedhart DM, Miltenburg M, Jordaens LJ. Morphology discrimination in implantable cardioverter-defibrillators: consistency of template match percentage during atrial tachyarrhythmias at different heart rates. Europace 2008;10:1060–6. [DOI] [PubMed] [Google Scholar]
- 123. Grönefeld GC. Discrimination of ventricular tachycardia from supraventricular tachycardia in implantable cardioverter defibrillators by automated electrogram morphology analysis: can leads finally replace the electrophysiologist? Europace 2008;10:1131–2. [DOI] [PubMed] [Google Scholar]
- 124. Shakibfar S, Krause O, Lund-Andersen C, Aranda A, Moll J, Andersen TOet al. . Predicting electrical storms by remote monitoring of implantable cardioverter-defibrillator patients using machine learning. Europace 2019;21:268–74. [DOI] [PubMed] [Google Scholar]
- 125. Narayan SM, Rogers AJ. Can machine learning disrupt the prediction of sudden death? J Am Coll Cardiol 2023;81:962–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Sammani A, van de Leur RR, Henkens M, Meine M, Loh P, Hassink RJet al. . Life-threatening ventricular arrhythmia prediction in patients with dilated cardiomyopathy using explainable electrocardiogram-based deep neural networks. Europace 2022;24:1645–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Balaban G, Halliday BP, Hammersley D, Rinaldi CA, Prasad SK, Bishop MJet al. . Left ventricular shape predicts arrhythmic risk in fibrotic dilated cardiomyopathy. Europace 2022;24:1137–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Attia ZI, Kapa S, Lopez-Jimenez F, McKie PM, Ladewig DJ, Satam Get al. . Screening for cardiac contractile dysfunction using an artificial intelligence-enabled electrocardiogram. Nat Med 2019;25:70–4. [DOI] [PubMed] [Google Scholar]
- 129. Attia ZI, Noseworthy PA, Lopez-Jimenez F, Asirvatham SJ, Deshmukh AJ, Gersh BJet al. . An artificial intelligence-enabled ECG algorithm for the identification of patients with atrial fibrillation during sinus rhythm: a retrospective analysis of outcome prediction. Lancet 2019;394:861–7. [DOI] [PubMed] [Google Scholar]
- 130. Khurshid S, Friedman S, Reeder C, Di Achille P, Diamant N, Singh Pet al. . ECG-Based Deep learning and clinical risk factors to predict atrial fibrillation. Circulation 2022;145:122–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Hermans BJM, Stoks J, Bennis FC, Vink AS, Garde A, Wilde AAMet al. . Support vector machine-based assessment of the T-wave morphology improves long QT syndrome diagnosis. Europace 2018;20:iii113–9. [DOI] [PubMed] [Google Scholar]
- 132. Kwon JM, Jeon KH, Kim HM, Kim MJ, Lim SM, Kim KHet al. . Comparing the performance of artificial intelligence and conventional diagnosis criteria for detecting left ventricular hypertrophy using electrocardiography. Europace 2020;22:412–9. [DOI] [PubMed] [Google Scholar]
- 133. Saglietto A, Gaita F, Blomstrom-Lundqvist C, Arbelo E, Dagres N, Brugada Jet al. . AFA-Recur: an ESC EORP AFA-LT registry machine-learning web calculator predicting atrial fibrillation recurrence after ablation. Europace 2023;25:92–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Rosier A, Mabo P, Temal L, Van Hille P, Dameron O, Deléger Let al. . Personalized and automated remote monitoring of atrial fibrillation. Europace 2016;18:347–52. [DOI] [PubMed] [Google Scholar]
- 135. Oto E, Okutucu S, Katircioglu-Öztürk D, Güvenir HA, Karaagaoglu E, Borggrefe Met al. . Predictors of sinus rhythm after electrical cardioversion of atrial fibrillation: results from a data mining project on the Flec-SL trial data set. Europace 2017;19:921–8. [DOI] [PubMed] [Google Scholar]
- 136. Okutucu S, Katircioglu-Öztürk D, Oto E, Güvenir HA, Karaagaoglu E, Oto Aet al. . Data mining experiments on the angiotensin II-antagonist in paroxysmal atrial fibrillation (ANTIPAF-AFNET 2) trial: ‘exposing the invisible’. Europace 2017;19:741–6. [DOI] [PubMed] [Google Scholar]
- 137. Costantino G, Falavigna G, Solbiati M, Casagranda I, Sun BC, Grossman SAet al. . Neural networks as a tool to predict syncope risk in the emergency department. Europace 2017;19:1891–5. [DOI] [PubMed] [Google Scholar]
- 138. Bacoyannis T, Ly B, Cedilnik N, Cochet H, Sermesant M. Deep learning formulation of electrocardiographic imaging integrating image and signal information with data-driven regularization. Europace 2021;23:i55–62. [DOI] [PubMed] [Google Scholar]
- 139. Vincent KP, Forsch N, Govil S, Joblon JM, Omens JH, Perry JCet al. . Atlas-based methods for efficient characterization of patient-specific ventricular activation patterns. Europace 2021;23:i88–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Luongo G, Vacanti G, Nitzke V, Nairn D, Nagel C, Kabiri Det al. . Hybrid machine learning to localize atrial flutter substrates using the surface 12-lead electrocardiogram. Europace 2022;24:1186–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Bhatia NK, Rogers AJ, Krummen DE, Hossainy S, Sauer W, Miller JMet al. . Termination of persistent atrial fibrillation by ablating sites that control large atrial areas. Europace 2020;22:897–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Ganesan P, Deb B, Feng R, Rodrigo M, Ruiperez-Campillo S, Rogers AJet al. . Quantifying a spectrum of clinical response in atrial tachyarrhythmias using spatiotemporal synchronization of electrograms. Europace 2023;25:euad055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Betts TR, Good WW, Melki L, Metzner A, Grace A, Verma Aet al. . Treatment of pathophysiologic propagation outside of the pulmonary veins in retreatment of atrial fibrillation patients: RECOVER AF study. Europace 2023;25:euad097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Krummen DE, Baykaner T, Schricker AA, Kowalewski CAB, Swarup V, Miller JMet al. . Multicentre safety of adding focal impulse and rotor modulation (FIRM) to conventional ablation for atrial fibrillation. Europace 2017;19:769–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Corrado C, Williams S, Roney C, Plank G, O’Neill M, Niederer S. Using machine learning to identify local cellular properties that support re-entrant activation in patient-specific models of atrial fibrillation. Europace 2021;23:i12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Toprak B, Brandt S, Brederecke J, Gianfagna F, Vishram-Nielsen JKK, Ojeda FMet al. . Exploring the incremental utility of circulating biomarkers for robust risk prediction of incident atrial fibrillation in European cohorts using regressions and modern machine learning methods. Europace 2023;25:812–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Loring Z, Mehrotra S, Piccini JP. Machine learning in ‘big data’: handle with care. Europace 2019;21:1284–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Loring Z, Mehrotra S, Piccini JP, Camm J, Carlson D, Fonarow GCet al. . Machine learning does not improve upon traditional regression in predicting outcomes in atrial fibrillation: an analysis of the ORBIT-AF and GARFIELD-AF registries. Europace 2020;22:1635–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Szymanski P, Leggeri I, Kautzner J, Fraser AG. The new European regulatory framework for medical devices: opportunities for engagement by electrophysiologists. Europace 2018;20:902–5. [DOI] [PubMed] [Google Scholar]
- 150. Choby AA, Clark AM. What costs matter? Rethinking social costs of new device technologies. Europace 2013;15:1538–9. [DOI] [PubMed] [Google Scholar]
- 151. Fattore G, Maniadakis N, Mantovani LG, Boriani G. Health technology assessment: what is it? Current status and perspectives in the field of electrophysiology. Europace 2011;13:ii49–53. [DOI] [PubMed] [Google Scholar]
- 152. Boriani G, Vitolo M, Svennberg E, Casado-Arroyo R, Merino JL, Leclercq Cet al. . Performance-based risk-sharing arrangements for devices and procedures in cardiac electrophysiology: an innovative perspective. Europace 2022;24:1541–7. [DOI] [PubMed] [Google Scholar]
- 153. Prinzen FW, Dagres N, Bollmann A, Arnar DO, Bove S, Camm Jet al. . Innovation in cardiovascular disease in Europe with focus on arrhythmias: current status, opportunities, roadblocks, and the role of multiple stakeholders. Europace 2018;20:733–8. [DOI] [PubMed] [Google Scholar]
- 154. Nagarajan VD, Lee SL, Robertus JL, Nienaber CA, Trayanova NA, Ernst Set al. . Artificial intelligence in the diagnosis and management of arrhythmias. Eur Heart J 2021;42:3904–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Somani S, Russak AJ, Richter F, Zhao S, Vaid A, Chaudhry Fet al. . Deep learning and the electrocardiogram: review of the current state-of-the-art. Europace 2021;23:1179–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Hermans ANL, Betz K, Verhaert DVM, den Uijl DW, Clerx K, Debie Let al. . 360° Virtual reality to improve patient education and reduce anxiety towards atrial fibrillation ablation. Europace 2023;25:855–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Shiraishi Y, Goto S, Niimi N, Katsumata Y, Goda A, Takei Met al. . Improved prediction of sudden cardiac death in patients with heart failure through digital processing of electrocardiography. Europace 2023;25:922–30. [DOI] [PMC free article] [PubMed] [Google Scholar]