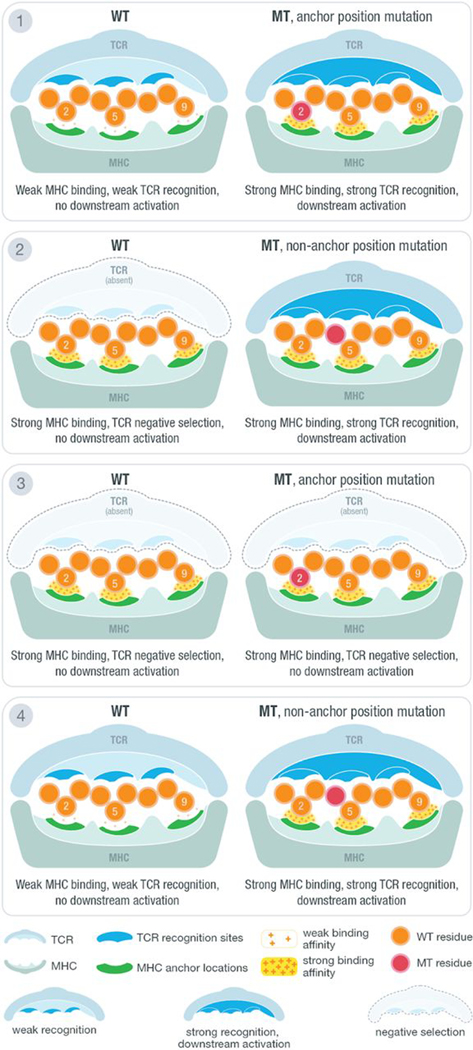
Fig. 1. Anchor and mutation position scenarios at the MHC-peptide-TCR interface.
Illustration of MHC-peptide-TCR interface using an example structure with anchors at positions 2, 5, and 9. At the contact interface between the peptide-loaded MHC and the recognizing T cell receptor, certain positions are responsible for anchoring the peptide to the MHC molecule and/or potentially being recognized by the TCR. The position of tumor-specific (“mutant”) amino acids relative to anchor positions and predicted binding affinity of MT and WT peptides produce four distinct scenarios for interpreting candidate neoantigens. Example TCR recognition sites are shown in blue, whereas MHC anchor locations are shown in green. The peptide residues are shown in orange, whereas the MT residue is marked with red. A yellow force field with varying density is used to illustrate binding strength between peptide and the MHC molecule. Three different cases of TCR recognition level are depicted, including self-recognizing TCR absent because of negative selection, weak-recognizing TCR due to weak MHC binding of presented peptide, and strong recognition of TCR triggering downstream activation of cytotoxic T cells.