Abstract
This article will discuss the past, present, and future of ventricular tachycardia ablation and the continuing contribution of the Europace journal as the platform for publication of milestone research papers in this field of ventricular tachycardia ablation.
Keywords: VT ablation, Energy, Technologies imaging
Introduction
Ventricular arrhythmias (VAs), an important cause of morbidity and mortality, are attributed to sudden cardiac death that used to be a terminal event until after the introduction of defibrillation therapy in the mid-20th century.1,2 Rapid developments have taken place since then in our understandings of the VAs and our ability to diagnose and treat them.3
Catheter ablation (CA) is currently considered the most effective non-pharmacological approach in reducing recurrence of VA.4 The ablation strategy, as well as its efficacy, is highly dependent on the accuracy of mapping of the substrate under investigation.4 Ventricular tachycardia (VT) ablation in structural heart disease has evolved from procedures based on mapping in VT to substrate mapping in sinus rhythm.3 Substrate mapping characterizes areas likely to support re-entry based on electrophysiological characteristics that can be determined during stable sinus or paced rhythm leading to efficient elimination of VTs.3 Additionally, ablation is now a valid therapeutic option for VF storm as well as for VAs in genetic syndromes such as Brugada and Long QT. Furthermore, with the advancements in mapping technology, we are now aware of many sites of origin of premature ventricular complexes (PVCs) other than the right ventricular outflow tract and fascicular VT.
Needless to say, understanding the substrate is the quintessence of successful VT ablations.5 Definite progress has been made in this direction with the introduction of high-density mapping, decrement-evoked potential-based VT substrate modification and hidden slow conduction analysis, multipolar catheters and the non-thermal pulse-field ablation system, etc. This article will discuss the past, present, and the future of VT ablation and the continuing contribution of the Europace journal as the platform for publication of milestone research papers in this field.
Energy options for ventricular tachycardia ablation
Among the energy options available for CA of VT, radiofrequency (RF) energy is often the first-line choice. However, RF energy is limited by the inability to create penetrating lesions to reach intramyocardial origins, emerging energy sources may improve the safety and efficacy of VT ablation.
Radiofrequency energy
For traditional single electrode discharge, the current density will continuously decay, which ensures that the deep tissue is heated, but also prevents excessive damage to the non-lesion. However, the pathological products such as blood clots, carbonized crusts, and microbubbles caused by electrical heating may trigger complications such as myocardial rupture and embolism. In the case of VT, the ventricular wall tends to be thick and scarred, so RF energy is often difficult to reach the ideal depth. The application of flushing irrigation catheters led to improved transmural injury, where cold saline irrigation can control lesion size by dispersing current density due to its conductive properties. When VT ablation is performed with irrigated RF catheters, the depth of lesion is ensured, and clot formation is reduced.6 In addition, the use of alternative perfusion agents such as half normal saline and glucose solutions has been reported to be able to produce deeper lesion.7 However, with low ion perfusion agents, reducing impedance may increase the risk of cardiac rupture and stroke.8 It has also been reported that bipolar ablation and simultaneous unipolar RF ablation can obtain deeper transmural injury and successfully terminate VT where unipolar ablation is unsuccessful.9,10 Given the potential risks of unpredictable myocardial injury with novel RF techniques, especially when increased current delivery is involved, randomized controlled studies are required. Moreover, the development of needle-tip catheters has been underway, the catheter can overcome the lack of intramural injury delivery while allowing for more refined mapping. Recently, a multicentre study showed acute and satisfactory mid-term control of difficult VTs.11
Alternative energy
In some VT cases where RF ablation fails due to catheter instability, cryoablation has better results because it improves catheter-myocardial contact. Berte et al.12 ablated a sheep model using a liquid nitrogen freezing system and showed better transmural injury without a significant increase in complications. Gordon et al.13 indicated that cryoenergy is a stable alternative energy for VT patients who have failed radiofrequency ablation. Ethanol ablation that can lead to effective substrate modification through coronary venous system is another option to ablate refractory VTs.14 Stereotactic radiotherapy (STAR) targeting the VT stromal region is a promising energy for VT patients, especially for those who cannot tolerate complex and prolonged cardiac procedure. This treatment is performed by non-invasive matrix labelling in combination with magnetic resonance cardiography to label the source of VT. The STAR can produce myocardial damage in localized areas of the ventricular myocardium. The Netherlands group published a registry prospective study that included 73 patients with ischaemic cardiomyopathy showed a reduction intreated VT episodes at the end of follow-up in 67 patients (87%). During the follow-up period, no treatment-related serious adverse events were observed.15 In addition, scientists have also proposed the use of proton beams, carbon beams, and other energies for VT ablation,16 but these non-invasive VT ablation methods require further studies on the long-term efficacy and potential effects of radiation on the heart and adjacent organs are still under investigation. Pulsed field ablation (PFA) is a novel, non-thermal modality by irreversibly electroporating cell membranes, which can spare collateral tissues damage. Koruth et al.17 demonstrated that in swine models, ventricular endocardial delivery of focal PFA is both safe and feasible. We look forward to more clinical trial data and in-depth studies of these new techniques.
Scar-related ventricular tachycardia
Ventricular tachycardia ablation guided by activation mapping
Programmed electrical stimulation (PES) and observations during initiation and termination have supported re-entry as mechanism of scar-related VT in patients with structural heart disease.18 Multielectrode mapping of ventricular activation during VT has enabled identification of critical sites at which ablation consistently interrupted the circuit in an animal infarct model.19 Mapping re-entrant VTs in humans is more complex, but the recognition of specific response characteristics following PES at re-entry circuit sites has allowed refinement in the localization of these sites relative to the circuit.20 These findings have paved the way for activation mapping guided VT-ablation targeting critical isthmus sites. Identification of these critical sites by entrainment mapping, confirmed by termination of VT during ablation, can be considered gold standard to verify any mapping criteria applied for substrate mapping during stable rhythm. Programmed electrical stimulation, activation, and entrainment mapping are tools to guide VT ablation but are also important to identify underlying mechanisms of VT and to further explore re-entry circuits in patients with different underlying heart diseases.
Modern multielectrode catheters allow for rapid electrogram acquisition with a potentially better accuracy for VT activation mapping because of the increased sensitivity for detection of low amplitude potentials in the nearfield. Mapping studies in post-infarct patients with slow, well-tolerated VT, in whom the entire VT circuit could be mapped, have provided new insights into complex circuit geometries.21 Of note, functional barriers to conduction in the isthmus were observed in 75%, confirming earlier high-resolution mapping studies of post-myocardial Infarction (MI) VT in a swine infarct model.22 Functional barriers are not always present during sinus rhythm (SR), highlighting limitations of substrate-based mapping, if performed during SR or slow pacing rates.
Detailed entrainment mapping has revealed that re-entry circuit isthmus sites of mappable post-MI VTs are more often located at the endocardium (62% of VTs), while the VT isthmus of mappable non-ischaemic cardiomyopathy (NICM) VTs could be identified at the endo- or epicardium in only 26%.23 Sequential endo- and epicardial high-density activation mapping of 83 stable VTs has demonstrated that only 7% of post-MI VTs and 28% of NICM VTs had the complete re-entry circuit confined to the endocardium or epicardium.24 The full diastolic pathway (between offset QRS and onset QRS) of clinical VTs, which allowed high-density activation mapping for at least 30 s, could be recorded in 42% of patients, more frequently in post-MI patients compared to NICM patients and in the majority (72%) from the endocardium.25 Recording of the full diastolic pathway followed by linear ablation at its narrowest site was associated with higher freedom from VT recurrence, compared to patients with partial diastolic pathway recording and substrate modification. These high-density activation or detailed entrainment mapping studies support a complex 3D activation with intramural components of re-entry circuits, difficult to detect by all current mapping techniques.
Of importance, activation and entrainment mapping have limitations and can only be applied in patients, who have mappable VT, defined as VT that18 is (reproducible) inducible,19 has stable conduction velocities and conduction paths, which are not altered by pacing, and20 is haemodynamically tolerated. Only a minority of patients have exclusively mappable VTs. The majority requires additional substrate mapping and ablation strategies to improve outcome after ablation.5 Whether fast or unstable VTs have similar features as mappable VT is unknown and verification of substrate mapping tools for these VTs difficult.
If ablation targets are not easily identifiable during SR or pacing manoeuvres, especially in patients with non-ischaemic cardiomyopathies, haemodynamic mechanical support (HMS) may allow activation and entrainment mapping of poorly tolerated, fast VT. Venoarterial extra corporeal membrane oxygenation (VA-ECMO) provides biventricular support and allows transseptal and transaortic mapping without electromagnetic interferences.26 Whether this approach will improve our understanding of 3D substrates and will facilitate targeted ablation, especially in non-ischaemic substrates, requires careful evaluation.
Substrate-based ventricular tachycardia mapping
Catheter ablation of VT based on activation mapping proved to terminate safely and effectively post-infarct VAs.20 Even though the investigated VT should be reproducibly inducible during the electrophysiology study and should be stable and slow enough to be properly mapped by this approach, no more than 30% of patients presenting with scar-related VT display inducible and stable VA.27 For this reason, substrate-based strategies have emerged as an alternative option to effectively map and ablate scar-related VT.28 From the identification and ablation of late potentials and local abnormal ventricular activities (LAVA) to scar dechanelling and scar homogenization,5 all these substrate-based strategies have greatly improved the understanding of VT pathophysiology and thus provide guidance in so-called unmappable ventricular circuits.29 However, these strategies differ for specific ablation endpoints5 and the extension of ventricular ablation,5 thus raising the questions on the best mapping strategy to adopt and in which clinical context.
In this regard, a meta-analysis published in this journal5 showed that substrate modification was associated with a decreased long-term combined risk of VA recurrence and all-cause mortality when compared to standard ablation of stable VT [risk ratio (RR) 0.57, 95% confidence interval (CI) 0.40–0.81]. In addition, the more extensive the ablation, the better the results. In fact, the long-term outcome was significantly improved when strategies based on complete substrate modifications were adopted vs. incomplete substrate modifications (RR 0.39, 95% CI 0.27–0.58).5 Of note, more than 70% of the patients included in the metanalysis had past medical history of ischaemic cardiomyopathy.
Explanation of these results is two-fold. On the one hand, the advantage of substrate modification might be related to the ablation of multiple scar channels that would be easily missed by activation mapping or standard ablation. On the other hand, extensive CA would be associated with a higher likelihood of eliminating circuits within the complexity of the ventricular scar. However long-term ablation results in patient with NICM seem to differ.30 Endo-epicardial homogenization of the ventricular scar may significantly increase the freedom from recurrent VT patients.31,32 In NICM patients, the outcomes are different. The reason should be sought in the septal and mid-myocardial distribution of the scar in patients affected by NICM that are typically associated with a greater risk of VT recurrence after ablation.33
Although the understanding of the ventricular substrate during VT ablation is paramount, the current knowledge and technology seem to fall behind. However, the progressive evolution of mapping technologies and ablation strategies will help clarify the best ablation approach in specific clinical scenarios and substrates. Meanwhile, extensive substrate ablation looks feasible in different clinical setting and, when necessary, the combination of both activation and substrate mapping strategies can still be of value in the most complex cases.
Catheter ablation of non-ischaemic cardiomyopathy ventricular tachycardia
The term NICM includes a heterogeneous group of cardiomyopathic states that are not due to coronary artery disease and with aetiologies ranging from genetic conditions to acquired inflammatory or infectious diseases.34 Despite these heterogeneities, NICM patients undergoing CA of VT share similarities in the substrate distribution and procedural approaches required to suppress VT.5,35 For instance, imaging and mapping studies have consistently documented a perivalvular distribution of the abnormal substrate responsible for VT in NICM and in patients with LV NICM, two patterns of scar distribution have been reported: a predominantly antero-septal (AS) pattern that has a high prevalence of intramural septal substrate extending to the periaortic region, and a predominantly infero-lateral (IL) pattern that is mostly characterized by basal LV free wall involvement greater in the epicardium compared to the endocardium. Delayed gadolinium-enhanced cardiac magnetic resonance imaging (CMR) is the gold standard for pre-procedural substrate characterization in NICM. Piers et al.36 have characterized the substrate distribution of NICM with pre-procedural CMR in 19 consecutive patients and highlighted the clinical importance of pre-procedural distinction between AS and IL substrate patterns owing to its impact on the procedural approaches, risks, and outcomes. Oloriz et al. have investigated the baseline 12-lead electrocardiogram (ECG) features in 108 patients with NICM undergoing VT ablation and found that the extent of unipolar low voltage in AS scar correlated with the PR interval and QRS duration, indicating involvement of the conduction system in the basal septum, whereas the extent of unipolar low voltage of the IL scar correlated with the mean voltage in the limb leads. In this study, a PR interval < 170 ms or QRS voltage < 0.6 mV or presence of lateral Q waves predicted IL substrate involvement with 92% sensitivity and 90% specificity, whereas a paced ventricular rhythm or PR > 230 ms or QRS > 170 ms or V3 lead R ≤ 0.3 mV had 92% and 81% sensitivity and specificity in predicting an AS substrate distribution.37 Patients with a predominantly AS substrate typically present with a basal-septal and often intramural substrate distribution and are best managed with endocardial-only procedures with minimal or no role for epicardial mapping and ablation. In these patients, substrate ablation carries risk of bystander injury to the proximal conduction system that can lead to acquired bundle branch block or complete heart block with implications for post-procedural need for device upgrade.38 Owing to the high prevalence of intramural scars in this group, bailout approaches such as bipolar RF ablation, simultaneous unipolar RF ablation, bipolar RF ablation, and use of lower ionic irrigant solutions or chemical ethanol ablation via arterial or venous septal perforator branches may be required.7,9,10,14
On the other hand, patients with a primarily IL substrate frequently present with a largely epicardial or combined epicardial and endocardial substrate and as such, tend to require epicardial instrumentation for mapping and ablation. The latter carries inherent risks associated with epicardial access together with risk of collateral injury to the major coronary arteries or phrenic nerve.39
Outcomes studies with CA of VT in NICM have overall demonstrated worse VT-free survival compared to ischaemic cardiomyopathy patients, which reflects the more challenging substrate distributions particularly in the AS scar subtype in the presence of an intramural scar distribution.40
Outcomes in specific subgroups
Ventricular tachycardia in arrhythmogenic right ventricular cardiomyopathy
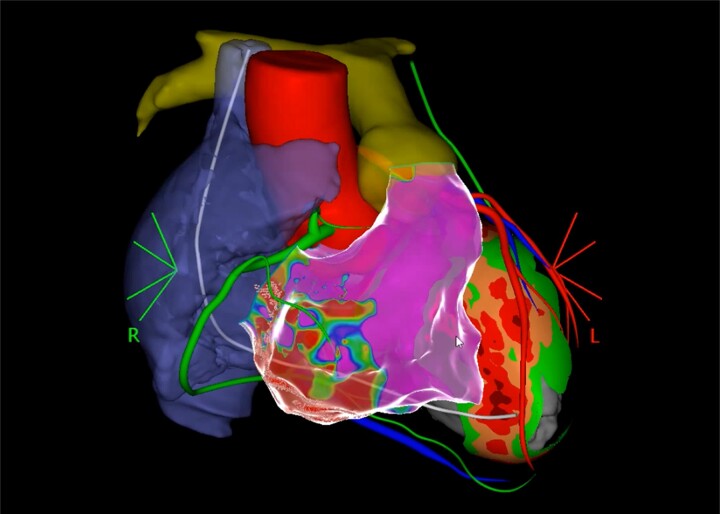
Arrhythmogenic right ventricular cardiomyopathy (ARVC) is genetically determined cardiomyopathy characterized by myocardial fibrofatty replacement that typically progresses from the epicardium to the endocardium. Such pathological substrate provides an ideal milieu for re-entrant VT.41,42 Similar to other types of NICM, the abnormal substrate in ARVC is perivalvular around the tricuspid annulus/pulmonic valve for RV-dominant subtypes, and perimitral/periaortic for LV-dominant subtypes.43 In ARVC patients, a combined endo-epicardial ablation approach has been consistently shown to provide excellent long-term outcomes in multiple observational studies and multicentre registries (Figure 1).44
Figure 1.
Co-registered 3D CT of a patient with ARVC. Left ventricle shows septal wall thinning, right atrium (blue), RCA (green), aorta and LAD (red), pulmonary artery (yellow), ICD lead (white), and left phrenic nerve (green). Electroanatomical reconstruction of the RV with infero-basal scar. LAD, left anterior descending coronary artery.
Ventricular tachycardia in cardiac sarcoidosis
Cardiac sarcoidosis is a peculiar type of NICM characterized by lymphocyte CD4+-mediated formation of non-necrotizing granulomas that results in reparative fibrosis that can lead to re-entrant VT. In addition to scar-mediated VT, patients with cardiac sarcoidosis may present also focal VTs due to triggered activity, particularly during acute inflammatory bouts. In a recent multicentre observational study, Siontis et al.45 have documented a negative prognostic impact of active inflammation detected on 18F-fluorodeoxyglucose positron emission tomography on VT recurrence. However, in some cases, immunosuppression alone may not be sufficient to control VT, and escalation of antiarrhythmic medications with or without adjunctive catheter ablation may be needed.46
Ablation of ventricular fibrillation in non-ischaemic disease states
Brugada syndrome
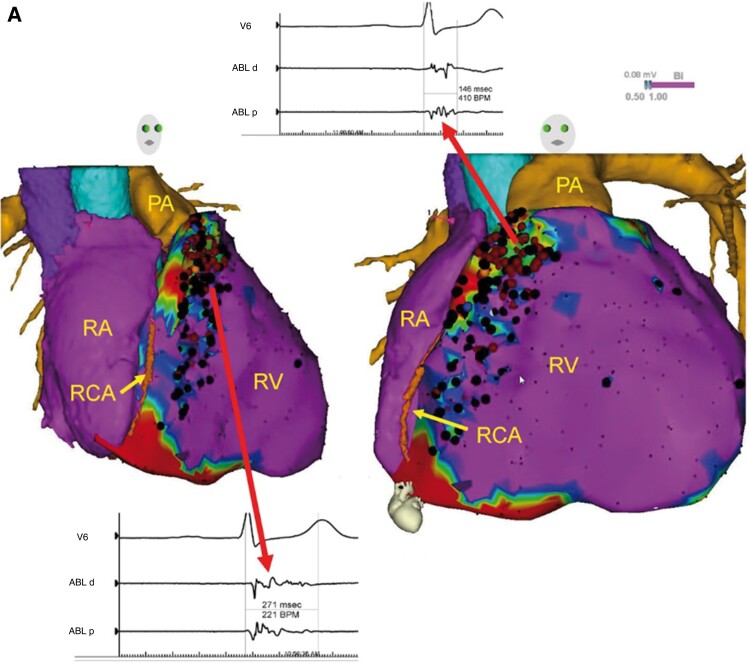
Brugada syndrome is a hereditary ion channel disease characterized by an elevated ST segment in leads V1 to V3 in the thoracic leads on the ECG, which often leads to polymorphic ventricular arrhythmias. Nademanee et al.47 found the presence of low-voltage zones and fragmentated potential zones on the epicardial surface of the right ventricular outflow tract in patients with Brugada syndrome (Figure 2), which can be ablated for ventricular fibrillation (VF) prevention. These results have been replicated and extended by Pappone et al.48 in a series of 135 patients. The authors confirmed a predominant RV outflow tract epicardial substrate location, and CA was associated with a high rate of complete ECG normalization and freedom from recurrent VF. A recent report from BRAVO (Brugada Ablation of VF Substrate Ongoing Multicenter Registry) suggested that catheter ablation treatment is safe and effective in preventing VF recurrence during a long-term follow-up period, achieving >95% 5 year VF-free survival in patients with highly symptomatic Brugada syndrome.49
Figure 2.
Epicardial substrate ablation in a patient with Brugada syndrome and history of VF. Purple represents bipolar voltage >1.5 mV. Fractionated potentials (arrows) are tagged with black dots, and a representative example is displayed. Adopted form Europace, Volume 22, Issue 3, March 2020, Pages 450–495.
Long QT syndrome
Haïssaguerre et al.50 first reported the relevance of VF triggers from the Purkinje system and right ventricular outflow tract in patients with hereditary Long QT syndrome (LQTS). In addition, Yap et al.51 reported that PVC trigger foci can also be distributed in the posterior left ventricular septum and outflow tract, the right ventricular septum, and the aortic valve region. In a recent study, Pappone et al.52 revealed that in high-risk LQTS patients, homogenization of the arrhythmogenic substrate that localized in the epicardium of the right ventricle successfully prevented malignant ventricular arrhythmia recurrences.
Early repolarization/J-point elevation syndrome and ventricular fibrillation
Early repolarization/J-wave syndrome (ERS/JWS) is usually a benign ECG finding, although a small subset of patients may develop VF. In a multicentre study in which substrate ablation, Purkinje PVC-triggered foci ablation, or a combination of both was performed to treat VF in early repolarization syndrome, 91% of patients had no recurrence of VF at a follow-up of (31 ± 26) months.53 Later, a multicentre study by Nademanee et al. increases the understanding of the pathophysiology of ERS/JWS. They found two distinct phenotypes in highly symptomatic patients with ERS/JWS: one with late depolarization abnormality that predominantly resides in the RV outflow tract and RV infero-lateral epicardium, and the other without substrates but with VF triggers that are associated with Purkinje sites. Ablation is effective in treating both phenotypes.54
Premature ventricular contraction ablation
Idiopathic VAs occur in patients without structural heart disease and comprise up to 10% of all VT. The predominant form is idiopathic PVCs accounting for ∼90% with the remainders manifesting themselves in the form of non-sustained or sustained idiopathic ventricular tachycardia (IVT). The prognostic implications of idiopathic PVC are considered favourable but there are conflicting reports.1,55,56 The most common source of origin are the left and right ventricular outflow tract (OT) accounting for almost 70% of all cases. The second most common form are fascicular VAs that derive from the left bundle branches and are probably also involved in papillary muscle and moderator band VA. Rarer locations are the mitral and tricuspid annulus.57
The most common mechanisms for idiopathic PVCs are triggered activity resulting from cAMP-mediated afterdepolarization and increased automaticity,58,59 rendering them inducible by adrenergic stimuli, increase intra-cellular Ca, or conversely suppressible by beta blocker, L-type Ca channel blockers, or adenosine.59
Patients should be treated once they become symptomatic, or the idiopathic PVCs/VT are thought to be involved in the deterioration of cardiac function.60 Development of left ventricular dysfunction can occur with a PVC burden as low as 10% with a higher risk at burdens of >20%.61 Elimination of the PVC often results in a significant improvement of the systolic function.62 Another important scenario is PVC-triggered ventricular fibrillation3 where elimination of the PVC may result in long-term freedom from malignant arrhythmia1.63
Correct identification of the site of origin guides the ablation strategy and helps predict the potential success rate. Twelve-lead ECG algorithms help to localize the site of origin with high accuracy.64,65
Outflow tract ectopy
The OT forms a complex anatomical structure166 that contains many of the most common sites of origin for ventricular ectopy in close proximity.62 The OT PVCs have been studied extensively and may be localized fairly accurately using surfaces ECG.64,65 All have inferior axis and negative vectors in aVR and aVL as a result of the cephalic origin. The morphology of lead V1 is of critical importance in localizing the site of origin along the sagittal plane of the heart. QS complexes in V1 with late transition (beyond V3) support right ventricular outflow tract (RVOT) origin while positive initial anterior forces resulting in rS or Rs complexes accompanied by earlier precordial transition represent more posterior sources. There are signature morphologies such a notched/qrS pattern in V1 in cases of right coronary cusp/left coronary cusp (RCC/LCC) commissure origin or a pattern break in V2 where the R wave in that lead is less positive than in V1 and V3 pseudo-delta patterns suggesting left ventricle (LV) summit origin.
Since some patients have no or very few PVC at the time of the procedure, intra-procedural PVC burden may be increased by atrial/ventricular pacing and/or administration of isoproterenol, caffeine, or epinephrine1.67 In addition, it has been shown that in most of these cases ablation guided mainly by pace-mapping may still be successful1.68 The long-term success of OT PVC ablation is very good,16,69–71 yet, some specific location and intramural foci still pose a significant challenge1.72 Such cases may require detailed mapping for multiple vantage points (both OTs, coronary venous system, and pulmonary artery), occasionally including epicardial mapping in order to locate a safe site for successful ablation.72 Non-standard techniques73 may be used to penetrate thick structures such simultaneous or sequential unipolar ablation from multiple sites,74 use of low-ionic irrigation solutions, bipolar ablation,75 needle ablation,76 and ethanol ablation or intracoronary ablation.77 An anecdotal report of using PFA for OT PVC has been published with encouraging result.78
The OT PVC ablation is safe with a consistently low rate of major complication.79 Nevertheless, it is important to consider the possibility of inadvertently ablating close to the coronary arteries. The risk is highest when ablation within the coronary cusps, the septal RVOT, above the pulmonary valve or epicardialy.66,80
Fascicular ectopy
Fascicular VAs are verapamil sensitive and display typical ECG signatures that reflect the involvement of the native conduction system. The most common type is the left postero-fascicular VT (LPF-VT) with a rather narrow QRS complex with right bundle branch block morphology, a rapid upstroke and left superior axis. Left antero-fascicular VT with an inferior axis is less common. The fascicular VA may be difficult to induce and pharmacological agents and/or ventricular stimulation may be required for induction. Once induced, the ablation success rate is high, and the LPF-VT can even be treated by targeting fragmented antegrade Purkinje potentials of the distal portion of the left fascicle if non-inducible with a long-term success rate exceeding 90%.81 Left upper septal fascicular VT (LUS-VT) is a rare variant that has a similar QRS morphology as in sinus rhythm and often only be induced by atrial stimulation.82,83 A network of fascicles, including a septal fascicle, can give rise to numerous different re-entries for fascicular VT.84,85
Intra-cavitary structures
The papillary muscles of the mitral valve, of the tricuspid valve, and the moderator band86 are increasingly recognized as important sites of origin for PVCs. Many of these PVCs have a common ECG feature of discordant axis in leads II and III64; in addition to location specific features. The LV papillary muscle (PM) displays right bundle (RB) morphology with a late precordial transition. Moderator band ectopy presents with left bundle (LB) morphology, late transition, superior axis, or negative lead III and positive II.64,87 The variability in their size, shape, and orientation poses specific challenges for ablation. This is best navigated with direct visualization using intracardiac echocardiography. Additional challenges are caterers stability and deep substrate. The former may be addressed by using a cryocatheter88 albeit at the cost of more limited manoeuvrability.
Drug therapy
Idiopathic PVC can be treated pharmacologically (flecainide, calcium channel blocker, beta blocker, or amiodarone), but ablation is the first-line treatment and is more effective in most cases.60,89 It is safe with success rates of 75–90%. If patients display different morphologies, success rates decline, and ablation of all PVC instead of the dominant form only results in lower PVC burden and better long-term ejection fraction (EF) improvement.90
Imaging technologies
Very frequently VTs are associated with structural changes of the heart—past ischaemias, infections, sutures, anastomosis, or malfunction of valves can lead to scarring of the ventricles that is a prerequisite for conduction heterogeneity and finally: ventricular tachycardias. This strong correlation of structural abnormalities and the functional symptom (VT) is the reason why imaging and VT ablation were always very closely linked. Over the past decades, both mapping and ablation but also imaging modalities have improved significantly and made these procedures more predictable, safer, and more successful.
At beginning of VT ablation, mainly fluoroscopy was supporting the anatomical orientation—resulting in relevant fluoroscopy times and -doses. Also when applying imaging modalities to VT ablations, the aspect of exposing radiation should be kept in mind—imaging like intracardiac echocardiography (ICE) and trans-esophagealechocardiography (TEE) is free of any potentially harming effects, MRI usually uses Gadolinium (which at least should be realized), and computed tomography (CT) can also expose the patient to substantial radiation—depending on the protocol.91 The use of MRI still is limited in clinical practice because of concerns regarding potential interactions with implanted devices (which most of VT patients have). Multiple studies clearly demonstrated that this concern is hardly ever justified and image quality and diagnostic yield are the only limitations to the widespread use of MRI in VT patients.92,93
The widespread use of 3D electroanatomical mapping systems certainly was the next major step in evolution of VT ablation. Detailed mapping became available and the re-entry circuits were no longer theory from the textbooks but became visible. Even better since imaging provided us further insights in the structural conditions of the heart: anticipating the need for epicardial access,94 planning the optimal access route, and even virtually creating lesion sets95 based on the scar pattern. Potential obstacles for ablation therapy became apparent like coronary arteries and epicardial fat96 with a good correlation between imaging (see Figure 1) and mapping results.97 Finally and logically, imaging was no longer only used to support the ablation procedure but really to guide it. Both CT and MRI can be used to assess the structural problem and to plan strategic lesions that after a step of registration can be deployed by the ablation catheter.98–100 It was demonstrated in these studies that even without induction and mapping of the tachycardia, the results were at least comparable when using a purely image-guided abolition of critical isthmus sites.
Finally, first attempts have been made to immediately deploy the ablation lesions within the site of image acquisition-MRI-guided ablation of typical atrial flutter already is feasible,101 and even VT ablations have been performed successfully in an animal study.102
In summary: the better we understand the underlying structural problem, the more targeted we can treat the arrhythmia. Multiple imaging modalities are available—all of them helping us in delivering safe and efficient therapy to our patients.
Ventricular tachycardia ablation: endpoints and clinical trials
Current guidelines60,103 indicate VT ablation in patients with structural heart disease and recurrent VT episodes causing implantable cardioverter-defibrillator (ICD) interventions.
Observational studies have shown that freedom from VT recurrence following ablation is associated with enhanced survival, decreased rate of worsening heart failure (wHF), and need for heart transplant.104 Also, it was found that an earlier access to VT ablation was related to lower recurrence rate.105,106
A survival benefit from CA, however, has not been yet demonstrated by prospective studies. The following issues concerning the role of VT ablation are still open questions to be addressed by prospective studies:
Timing: prophylactic at the time of ICD implant, after the first shock, after recurrent VT episodes, or even after electrical storm.
Does the prevention of VT impact survival, or affect the occurrence of heart failure?
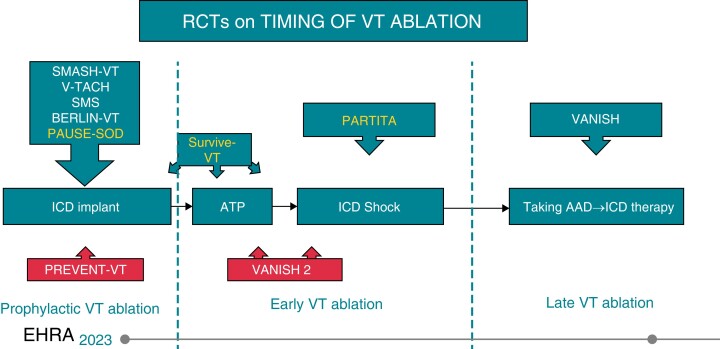
Over the last 10 years, several studies focused on the effects of preventive catheter ablation, randomizing patients at the time of ICD implant to an ablative treatment and to a medical treatment arm107–111 (Figure 3). The uniform finding was an overall reduction of recurrent VT episodes and a decreased incidence of appropriate shocks, without significant effects on overall survival. Although a possible indication to preventive VT ablation at the time of ICD implant has been foreseen in selected cases by the latest ESC VT ablation guidelines,60 it is unlikely that this might become a widespread strategy. Many patients after the ICD implant might never have a recurrent arrhythmia pattern, needless to add, a uniform strategy of ablation at the time of or shortly after ICD implant will prove unpractical and economically unfeasible.
Figure 3.
Randomized controlled trial describing the timing for VT ablation.
Two recently published prospective randomized studies provide important information on the outcome following an early ablation strategy at ICD intervention: The SurviveVT112 and the PARTITA.113
The former prospectively compared the outcome of a population of 144 with recurrent post-infarction VT after an ICD implant, 71 randomized into an early ablation strategy, and the remaining 73 into an antiarrhythmic drug (AAD) treatment arm, mostly including amiodarone and to a lesser extent sotalol. Although it did not prove a short-term (2 year follow-up) survival benefit from CA, it demonstrated a significantly higher incidence of severe treatment-related complications in the AAD arm: 15/73 patients (20%) required early hospital admissions (mostly within 4 months) due to recurrent untreated or incessant slow VTs causing hospitalization and requiring subsequent CA and the discontinuation of the ongoing treatment. Arrhythmia recurrence and hospitalization occurred to a significantly lesser extent among patients that had undergone early ablation. Therefore, according to these findings, ablation performs better then drugs on the long run.
The PARTITA trial was designed to verify the prognostic impact of early VT ablation after the first ICD shock on the endpoints of mortality and wHF and to provide data on the natural history of VT following ICD implantation, including the identification of specific arrhythmia patterns that may predict a subsequent shock.
In this multicentre prospective two-stage, the rate of the primary endpoint of death and wHF hospitalization was 4.3% in the ablation group vs. 41.7% in the control group with a relative risk reduction of 89%.
Catheter ablation was also associated with lower recurrence of VTs treated by shock and with lower mortality. These findings, so far unique among the accomplished clinical studies on VT, support an early (after the first appropriate shock) ablation, both in patients with ischaemic and non-ischaemic dilated cardiomyopathy.
Also, it is worth mentioning that, due to the uniform implementation of modern ICD programming settings (prolonged detection times and high rate cut-offs),114 the rate of shock was lower than originally expected, but the negative prognostic value of an untreated active arrhythmia pattern is strengthened by these findings. Treatments with ATP were also linked to subsequent shock, and a quantitative analysis of this issue is under evaluation.
Far from being over, the quest is moving to further prospective studies to provide solid and definitive evidence that elimination of VT recurrence by ablation favourably affects the natural history of HF and prolongs survival in patients with heart disease. Focus on: patient selection (active arrhythmia pattern and low EF) standardization of ICD programming according to modern criteria rigorous compliance to state-of-the-art ablation protocols and homogeneous procedure endpoints.
Contributor Information
Andrea Natale, Department of Electrophysiology, Texas Cardiac Arrhythmia Institute, 3000 N. I-35, Suite 720, Austin, TX 78705, USA.
Katja Zeppenfeld, Department of Cardiology, Willem Einthoven Center of Arrhythmia Research and Management, Leiden University Medical Center, Leiden, the Netherlands.
Paolo Della Bella, Department of Cardiac Electrophysiology and Arrhythmology, San Raffaele University Hospital, Milan, Italy.
Xu Liu, Shanghai Chest Hospital, Shanghai Jiao Tong University, Shanghai, China.
Avi Sabbag, Sheba Medical Center, Tel HaShomer, Israel and the Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel.
Pasquale Santangeli, Cardiac Electrophysiology and Pacing, Cleveland Clinic, Cleveland, OH, USA.
Philipp Sommer, Heart and Diabetes Center NRW, Ruhr University Bochum, Bad Oeynhausen, Germany.
Christian Sticherling, University Hospital Basel, University of Basel, Basel, Switzerland.
Xiaodong Zhang, Montefiore Health System, Einstein Medical School, New York, USA.
Luigi Di Biase, Department of Electrophysiology, Texas Cardiac Arrhythmia Institute, 3000 N. I-35, Suite 720, Austin, TX 78705, USA; Montefiore Health System, Einstein Medical School, New York, USA.
Funding
C.S. is a member of Medtronic Advisory Board Europe and Boston Scientitic Advisory Board Europe, received educational grants from Biosense Webster and Biotronik and a research grant from the European Union’s FP7 programme and Biosense Webster, and lecture and consulting fees from Abbott, Medtronic, Biosense-Webster, Boston Scientific, Microport, and Biotronik all outside the submitted work This research has not been funded directly by any organization.
References
- 1. Kahle A-K, Jungen C, Alken F-A, Scherschel K, Willems S, Pürerfellner Het al. . Management of ventricular tachycardia in patients with ischaemic cardiomyopathy: contemporary armamentarium. Europace 2022;24:538–51. [DOI] [PubMed] [Google Scholar]
- 2. Guandalini GS, Liang JJ, Marchlinski FE. Ventricular tachycardia ablation: past, present, and future perspectives. JACC Clin Electrophysiol 2009;5:1363–83. [DOI] [PubMed] [Google Scholar]
- 3. Cronin EM, Bogun FM, Maury P, Peichl P, Chen M, Namboodiri Net al. . 2019 HRS/EHRA/APHRS/LAHRS expert consensus statement on catheter ablation of ventricular arrhythmias. Europace 2019;21:1143–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bianchi S, Cauti FM. Ablation of ventricular tachycardia in 2021. Eur Heart J Suppl 2021;23:E25–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Briceño DF, Romero J, Villablanca PA, Londoño A, Diaz JC, Maraj Iet al. . Long-term outcomes of different ablation strategies for ventricular tachycardia in patients with structural heart disease: systematic review and meta-analysis. Europace 2018;20:104–15. [DOI] [PubMed] [Google Scholar]
- 6. Marchlinski FE, Haffajee CI, Beshai JF, Dickfeld TL, Gonzalez MD, Hsia HHet al. . Long-term success of irrigated radiofrequency catheter ablation of sustained ventricular tachycardia: post-approval THERMOCOOL VT trial. J Am Coll Cardiol 2016;67:674–83. [DOI] [PubMed] [Google Scholar]
- 7. Nguyen DT, Olson M, Zheng L, Barham W, Moss JD, Sauer WH. Effect of irrigant characteristics on lesion formation after radiofrequency energy delivery using ablation catheters with actively cooled tips. J Cardiovasc Electrophysiol 2015;26:792–8. [DOI] [PubMed] [Google Scholar]
- 8. Shapira-Daniels A, Barkagan M, Rottmann M, Sroubek J, Tugal D, Carlozzi MAet al. . Modulating the baseline impedance: an adjunctive technique for maximizing radiofrequency lesion dimensions in deep and intramural ventricular substrate. Circ Arrhythm Electrophysiol 2019;12:e007336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gizurarson S, Spears D, Sivagangabalan G, Farid T, Ha AC, Massé Set al. . Bipolar ablation for deep intra-myocardial circuits: human ex vivo development and in vivo experience. Europace 2014;16:1684–8. [DOI] [PubMed] [Google Scholar]
- 10. Yang J, Liang J, Shirai Y, Muser D, Garcia FC, Callans DJet al. . Outcomes of simultaneous unipolar radiofrequency catheter ablation for intramural septal ventricular tachycardia in nonischemic cardiomyopathy. Heart Rhythm 2019;16:863–70. [DOI] [PubMed] [Google Scholar]
- 11. Packer DL, Wilber DJ, Kapa S, Dyrda K, Nault I, Killu AMet al. . Ablation of refractory ventricular tachycardia using intramyocardial needle delivered heated saline-enhanced radiofrequency energy: a first-in-man feasibility trial. Circ Arrhythm Electrophysiol 2022;15:e010347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Berte B, Sacher F, Wielandts JY, Mahida S, Pillois X, Weerasooriya Ret al. . A new cryoenergy for ventricular tachycardia ablation: a proof-of-concept study. Europace 2017;19:1401–7. [DOI] [PubMed] [Google Scholar]
- 13. Gordon JP, Liang JJ, Pathak RK, Zado ES, Garcia FC, Hutchinson MDet al. . Percutaneous cryoablation for papillary muscle ventricular arrhythmias after failed radiofrequency catheter ablation. J Cardiovasc Electrophysiol 2018;29:1654–63. [DOI] [PubMed] [Google Scholar]
- 14. Valderrábano M, Fuentes Rojas SC, Lador A, Patel A, Schurmann PA, Tapias Cet al. . Substrate ablation by multivein, multiballoon coronary venous ethanol for refractory ventricular tachycardia in structural heart disease. Circulation 2022;146:1644–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. van der Ree MH, Dieleman EMT, Visser J, Planken RN, Boekholdt SM, de Bruin-Bon RHAet al. . Non-invasive stereotactic arrhythmia radiotherapy for ventricular tachycardia: results of the prospective STARNL-1 trial. Europace 2023;25:1015–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hohmann S, Deisher AJ, Suzuki A, Konishi H, Rettmann ME, Merrell KWet al. . Left ventricular function after noninvasive cardiac ablation using proton beam therapy in a porcine model. Heart Rhythm 2019;16:1710–9. [DOI] [PubMed] [Google Scholar]
- 17. Koruth JS, Kuroki K, Iwasawa J, Viswanathan R, Brose R, Buck EDet al. . Endocardial ventricular pulsed field ablation: a proof-of-concept preclinical evaluation. Europace 2020;22:434–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wellens HJ, Schuilenburg RM, Durrer D. Electrical stimulation of the heart in patients with ventricular tachycardia. Circulation 1972;46:216–26. [DOI] [PubMed] [Google Scholar]
- 19. El-Sherif N, Mehra R, Gough WB, Zeiler RH. Reentrant ventricular arrhythmias in the late myocardial infarction period. Interruption of reentrant circuits by cryothermal techniques. Circulation 1983;68:644–56. [DOI] [PubMed] [Google Scholar]
- 20. Stevenson WG, Khan H, Sager P, Saxon LA, Middlekauff HR, Natterson PDet al. . Identification of reentry circuit sites during catheter mapping and radiofrequency ablation of ventricular tachycardia late after myocardial infarction. Circulation 1993;88:1647–70. [DOI] [PubMed] [Google Scholar]
- 21. Martin R, Maury P, Bisceglia C, Wong T, Estner H, Meyer Cet al. . Characteristics of scar-related ventricular tachycardia circuits using ultra-high-density mapping: a multi-center study. Circ Arrhythmia Electrophysiol 2018;11:e006569. [DOI] [PubMed] [Google Scholar]
- 22. Anter E, Tschabrunn CM, Buxton AE, Josephson ME. High-resolution mapping of postinfarction reentrant ventricular tachycardia: electrophysiological characterization of the circuit. Circulation 2016;134:314–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shirai Y, Liang JJ, Santangeli P, Arkles JS, Schaller RD, Supple GEet al. . Comparison of the ventricular tachycardia circuit between patients with ischemic and nonischemic cardiomyopathies: detailed characterization by entrainment. Circ Arrhythmia Electrophysiol 2019;12:e007249. [DOI] [PubMed] [Google Scholar]
- 24. Tung R, Raiman M, Liao H, Zhan X, Chung FP, Nagel Ret al. . Simultaneous endocardial and epicardial delineation of 3D reentrant ventricular tachycardia. J Am Coll Cardiol 2020;75:884–97. [DOI] [PubMed] [Google Scholar]
- 25. Hadjis A, Frontera A, Limite LR, Bisceglia C, Bognoni L, Foppoli Let al. . Complete electroanatomic imaging of the diastolic pathway is associated with improved freedom from ventricular tachycardia recurrence. Circ Arrhythmia Electrophysiol 2020;13:e008651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Della Bella P, Radinovic A, Limite LR, Baratto F. Mechanical circulatory support in the management of life-threatening arrhythmia. Europace 2021;23:1166–78. [DOI] [PubMed] [Google Scholar]
- 27. Zeppenfeld K, Stevenson WG. Ablation of ventricular tachycardia in patients with structural heart disease. Pacing Clin Electrophysiol 2008;31:358–74. [DOI] [PubMed] [Google Scholar]
- 28. Bourier F, Martin R, Martin CA, Takigawa M, Kitamura T, Frontera Aet al. . Is it feasible to offer “targeted ablation” of ventricular tachycardia circuits with better understanding of isthmus anatomy and conduction characteristics? Europace 2019;21:I27–33. [DOI] [PubMed] [Google Scholar]
- 29. Acosta J, Cabanelas N, Penela D, Fernández-Armenta J, Andreu D, Borràs Ret al. . Long-term benefit of first-line peri-implantable cardioverter-defibrillator implant ventricular tachycardia-substrate ablation in secondary prevention patients. Europace 2017;19:976–82. [DOI] [PubMed] [Google Scholar]
- 30. Proietti R, Essebag V, Beardsall J, Hache P, Pantano A, Wulffhart Zet al. . Substrate-guided ablation of haemodynamically tolerated and untolerated ventricular tachycardia in patients with structural heart disease: effect of cardiomyopathy type and acute success on long-term outcome. Europace 2015;17:461–7. [DOI] [PubMed] [Google Scholar]
- 31. Di Biase L, Burkhardt JD, Lakkireddy D, Carbucicchio C, Mohanty S, Mohanty Pet al. . Ablation of stable VTs versus substrate ablation in ischemic cardiomyopathy: the VISTA randomized multicenter trial. J Am Coll Cardiol 2015;66:2872–82. [DOI] [PubMed] [Google Scholar]
- 32. Quinto L, Sanchez P, Alarcón F, Garre P, Zaraket F, Prat-Gonzalez Set al. . Cardiac magnetic resonance to predict recurrences after ventricular tachycardia ablation: septal involvement, transmural channels, and left ventricular mass. Europace 2021;23:1437–45. [DOI] [PubMed] [Google Scholar]
- 33. Di Biase L, Santangeli P, Burkhardt DJ, Bai R, Mohanty P, Carbucicchio Cet al. . Endo-epicardial homogenization of the scar versus limited substrate ablation for the treatment of electrical storms in patients with ischemic cardiomyopathy. J Am Coll Cardiol 2012;60:132–41. [DOI] [PubMed] [Google Scholar]
- 34. Elliott P, Andersson B, Arbustini E, Bilinska Z, Cecchi F, Charron Pet al. . Classification of the cardiomyopathies: a position statement from the european Society of Cardiology working group on myocardial and pericardial diseases. Eur Heart J 2008;29:270–6. [DOI] [PubMed] [Google Scholar]
- 35. Nakahara S, Tung R, Ramirez RJ, Michowitz Y, Vaseghi M, Buch Eet al. . Characterization of the arrhythmogenic substrate in ischemic and nonischemic cardiomyopathy. J Am Coll Cardiol 2010;55:2355–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Piers SR, Tao Q, van Huls van Taxis CF, Schalij MJ, van der Geest RJ, Zeppenfeld K. Contrast-enhanced MRI-derived scar patterns and associated ventricular tachycardias in nonischemic cardiomyopathy: implications for the ablation strategy. Circ Arrhythm Electrophysiol 2013;6:875–83. [DOI] [PubMed] [Google Scholar]
- 37. Oloriz T, Wellens HJ, Santagostino G, Trevisi N, Silberbauer J, Peretto Get al. . The value of the 12-lead electrocardiogram in localizing the scar in non-ischaemic cardiomyopathy. Europace 2016;18:1850–9. [DOI] [PubMed] [Google Scholar]
- 38. Oloriz T, Silberbauer J, Maccabelli G, Mizuno H, Baratto F, Kirubakaran Set al. . Catheter ablation of ventricular arrhythmia in nonischemic cardiomyopathy: anteroseptal versus inferolateral scar sub-types. Circ Arrhythm Electrophysiol 2014;7:414–23. [DOI] [PubMed] [Google Scholar]
- 39. Della Bella P, Brugada J, Zeppenfeld K, Merino J, Neuzil P, Maury Pet al. . Epicardial ablation for ventricular tachycardia: a European multicenter study. Circ Arrhythm Electrophysiol 2011;4:653–9. [DOI] [PubMed] [Google Scholar]
- 40. Liang JJ, Santangeli P, Callans DJ. Long-term outcomes of ventricular tachycardia ablation in different types of structural heart disease. Arrhythm Electrophysiol Rev 2015;4:177–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yamada S, Hsiao YW, Chang SL, Lin YJ, Lo LW, Chung FPet al. . Circulating microRNAs in arrhythmogenic right ventricular cardiomyopathy with ventricular arrhythmia. Europace 2018;20:f37–45. [DOI] [PubMed] [Google Scholar]
- 42. Kirubakaran S, Bisceglia C, Silberbauer J, Oloriz T, Santagostino G, Yamase Met al. . Characterization of the arrhythmogenic substrate in patients with arrhythmogenic right ventricular cardiomyopathy undergoing ventricular tachycardia ablation. Europace 2017;19:1049–62. [DOI] [PubMed] [Google Scholar]
- 43. Marchlinski FE, Zado E, Dixit S, Gerstenfeld E, Callans DJ, Hsia Het al. . Electroanatomic substrate and outcome of catheter ablative therapy for ventricular tachycardia in setting of right ventricular cardiomyopathy. Circulation 2004;110:2293–8. [DOI] [PubMed] [Google Scholar]
- 44. Garcia FC, Bazan V, Zado ES, Ren J-F, Marchlinski FE. Epicardial substrate and outcome with epicardial ablation of ventricular tachycardia in arrhythmogenic right ventricular cardiomyopathy/dysplasia. Circulation 2009;120:366–75. [DOI] [PubMed] [Google Scholar]
- 45. Siontis KC, Santangeli P, Muser D, Marchlinski FE, Zeppenfeld K, Hoogendoorn JCet al. . Outcomes associated with catheter ablation of ventricular tachycardia in patients with cardiac sarcoidosis. JAMA Cardiol 2022;7:175–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Naruse Y, Sekiguchi Y, Nogami A, Okada H, Yamauchi Y, Machino Tet al. . Systematic treatment approach to ventricular tachycardia in cardiac sarcoidosis. Circ Arrhythm Electrophysiol 2014;7:407–13. [DOI] [PubMed] [Google Scholar]
- 47. Nademanee K, Veerakul G, Chandanamattha P, Chaothawee L, Ariyachaipanich A, Jirasirirojanakorn Ket al. . Prevention of ventricular fibrillation episodes in Brugada syndrome by catheter ablation over the anterior right ventricular outflow tract epicardium. Circulation 2011;123:1270–9. [DOI] [PubMed] [Google Scholar]
- 48. Pappone C, Brugada J, Vicedomini G, Ciconte G, Manguso F, Saviano Met al. . Electrical substrate elimination in 135 consecutive patients with Brugada syndrome. Circ Arrhythm Electrophysiol 2017;10:e005053. [DOI] [PubMed] [Google Scholar]
- 49. Nademanee K, Chung FP, Sacher F, Nogami A, Nakagawa H, Jiang Cet al. . Long-term outcomes of Brugada substrate ablation: a report from BRAVO (Brugada Ablation of VF Substrate Ongoing Multicenter Registry). Circulation 2023;147:1568–78. [DOI] [PubMed] [Google Scholar]
- 50. Haïssaguerre M, Extramiana F, Hocini M, Cauchemez B, Jaïs P, Cabrera JAet al. . Mapping and ablation of ventricular fibrillation associated with long-QT and Brugada syndromes. Circulation 2003;108:925–8. [DOI] [PubMed] [Google Scholar]
- 51. Yap J, Tan VH, Hsu LF, Liew R. Catheter ablation of ventricular fibrillation storm in a long QT syndrome genotype carrier with normal QT interval. Singapore Med J 2013;54:e1-4. [PubMed] [Google Scholar]
- 52. Pappone C, Ciconte G, Anastasia L, Gaita F, Grant E, Micaglio Eet al. . Right ventricular epicardial arrhythmogenic substrate in long-QT syndrome patients at risk of sudden death. Europace 2023;25:948–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Haïssaguerre M, Derval N, Sacher F, Jesel L, Deisenhofer I, de Roy Let al. . Sudden cardiac arrest associated with early repolarization. N Engl J Med 2008;358:2016–23. [DOI] [PubMed] [Google Scholar]
- 54. Nademanee K, Haissaguerre M, Hocini M, Nogami A, Cheniti G, Duchateau Jet al. . Mapping and ablation of ventricular fibrillation associated with early repolarization syndrome. Circulation 2019;140:1477–90. [DOI] [PubMed] [Google Scholar]
- 55. Dukes JW, Dewland TA, Vittinghoff E, Mandyam MC, Heckbert SR, Siscovick DSet al. . Ventricular ectopy as a predictor of heart failure and death. J Am Coll Cardiol 2015;66:101–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Scorza R, Jonsson M, Friberg L, Rosenqvist M, Frykman V. Prognostic implication of premature ventricular contractions in patients without structural heart disease. Europace 2023;25:517–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Muser D, Santangeli P, Liang JJ. Mechanisms of ventricular arrhythmias and implications for catheter ablation. Card Electrophysiol Clin 2022;14:547–58. [DOI] [PubMed] [Google Scholar]
- 58. Lerman BB. Mechanism, diagnosis, and treatment of outflow tract tachycardia. Nat Rev Cardiol 2015;12:597–608. [DOI] [PubMed] [Google Scholar]
- 59. Marcus GM. Evaluation and management of premature ventricular complexes. Circulation 2020;141:1404–18. [DOI] [PubMed] [Google Scholar]
- 60. Zeppenfeld K, Tfelt-Hansen J, de Riva M, Winkel BG, Behr ER, Blom NAet al. . 2022 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J 2022:3997–4126.36017572 [Google Scholar]
- 61. Baman TS, Lange DC, Ilg KJ, Gupta SK, Liu TY, Alguire Cet al. . Relationship between burden of premature ventricular complexes and left ventricular function. Heart Rhythm 2010;3:865–9. [DOI] [PubMed] [Google Scholar]
- 62. Latchamsetty R, Yokokawa M, Morady F, Kim HM, Mathew S, Tilz Ret al. . Multicenter outcomes for catheter ablation of idiopathic premature ventricular complexes. JACC Clin Electrophysiol 2015;1:116–23. [DOI] [PubMed] [Google Scholar]
- 63. Knecht S, Sacher F, Wright M, Hocini M, Nogami A, Arentz Tet al. . Long-term follow-up of idiopathic ventricular fibrillation ablation: a multicenter study. J Am Coll Cardiol 2009;54:522–8. [DOI] [PubMed] [Google Scholar]
- 64. Enriquez A, Baranchuk A, Briceno D, Saenz L, Garcia F. How to use the 12-lead ECG to predict the site of origin of idiopathic ventricular arrhythmias. Heart Rhythm 2019;16:1538–44. [DOI] [PubMed] [Google Scholar]
- 65. Yamada T. Twelve-lead electrocardiographic localization of idiopathic premature ventricular contraction origins. J Cardiovasc Electrophysiol 2019;30:2603–17. [DOI] [PubMed] [Google Scholar]
- 66. Asirvatham SJ. Correlative anatomy for the invasive electrophysiologist: outflow tract and supravalvar arrhythmia. J Cardiovasc Electrophysiol 2009;20:955–68. [DOI] [PubMed] [Google Scholar]
- 67. Hasebe H, Yoshida K, Furuyashiki Y, Nogami A, Ieda M. Oral caffeine intake amplifies the effect of isoproterenol in patients with frequent premature ventricular contractions. Europace 2020;22:1261–9. [DOI] [PubMed] [Google Scholar]
- 68. Shirai Y, Liang JJ, Santangeli P, Supple GE, Riley MP, Garcia FCet al. . Catheter ablation of premature ventricular complexes with low intraprocedural burden guided exclusively by pace-mapping. J Cardiovasc Electrophysiol 2019;30:2326–33. [DOI] [PubMed] [Google Scholar]
- 69. Krittayaphong R, Sriratanasathavorn C, Dumavibhat C, Pumprueg S, Boonyapisit W, Pooranawattanakul Set al. . Electrocardiographic predictors of long-term outcomes after radiofrequency ablation in patients with right-ventricular outflow tract tachycardia. Europace 2006;8:601–6. [DOI] [PubMed] [Google Scholar]
- 70. Coggins DL, Lee RJ, Sweeney J, Chein WW, Van Hare G, Epstein Let al. . Radiofrequency catheter ablation as a cure for idiopathic tachycardia of both left and right ventricular origin. J Am Coll Cardiol 1994;23:1333–41. [DOI] [PubMed] [Google Scholar]
- 71. Ábrahám P, Ambrus M, Herczeg S, Szegedi N, Nagy KV, Salló Zet al. . Similar outcomes with manual contact force ablation catheters and traditional catheters in the treatment of outflow tract premature ventricular complexes. Europace 2021;23:596–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Hanson M, Futyma P, Bode W, Liang JJ, Tapia C, Adams Cet al. . Catheter ablation of intramural outflow tract premature ventricular complexes: a multicentre study. Europace 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Neira V, Santangeli P, Futyma P, Sapp J, Valderrabano M, Garcia Fet al. . Ablation strategies for intramural ventricular arrhythmias. Heart Rhythm 2020;7:1176–84. [DOI] [PubMed] [Google Scholar]
- 74. Yamada T, Maddox WR, McElderry HT, Doppalapudi H, Plumb VJ, Kay GN. Radiofrequency catheter ablation of idiopathic ventricular arrhythmias originating from intramural foci in the left ventricular outflow tract: efficacy of sequential versus simultaneous unipolar catheter ablation. Circ Arrhythm Electrophysiol 2015;8:344–52. [DOI] [PubMed] [Google Scholar]
- 75. Kany S, Alken FA, Schleberger R, Baran J, Luik A, Haas Aet al. . Bipolar ablation of therapy-refractory ventricular arrhythmias: application of a dedicated approach. Europace 2022;24:959–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Stevenson WG, Tedrow UB, Reddy V, AbdelWahab A, Dukkipati S, John RMet al. . Infusion needle radiofrequency ablation for treatment of refractory ventricular arrhythmias. J Am Coll Cardiol 2019;73:1413–25. [DOI] [PubMed] [Google Scholar]
- 77. Romero J, Shivkumar K, Valderrabano M, Diaz JC, Alviz I, Briceno Det al. . Modern mapping and ablation techniques to treat ventricular arrhythmias from the left ventricular summit and interventricular septum. Heart Rhythm 2020;17:1609–20. [DOI] [PubMed] [Google Scholar]
- 78. Schmidt B, Chen S, Tohoku S, Bordignon S, Bologna F, Chun KRJ. Single shot electroporation of premature ventricular contractions from the right ventricular outflow tract. Europace 2021;24:597–597. [DOI] [PubMed] [Google Scholar]
- 79. Fichtner S, Senges J, Hochadel M, Tilz R, Willems S, Eckardt Let al. . Safety and efficacy in ablation of premature ventricular contraction: data from the German ablation registry. Clin Res Cardiol 2017;106:49–57. [DOI] [PubMed] [Google Scholar]
- 80. Lavalle C, Mariani MV, Piro A, Straito M, Severino P, Della Rocca DGet al. . Electrocardiographic features, mapping and ablation of idiopathic outflow tract ventricular arrhythmias. J Interv Card Electrophysiol 2020;57:207–18. [DOI] [PubMed] [Google Scholar]
- 81. Wei HQ, Liao Z, Liang Y, Fang X, Liao H, Deng Het al. . Electrophysiological characteristics and long-term outcome of substrate-based catheter ablation for left posterior fascicular ventricular tachycardia targeting fragmented antegrade Purkinje potentials during sinus rhythm. Europace 2023;25:1008–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Guo XG, Liu X, Zhou GB, Sun Q, Yang JD, Luo Bet al. . Clinical, electrocardiographic, and electrophysiological characteristics of left upper septal fascicular ventricular tachycardia. Europace 2018;20:673–81. [DOI] [PubMed] [Google Scholar]
- 83. Li MM, Wu XY, Jiang CX, Ning M, Sang CH, Li SNet al. . Fascicular ventricular tachycardia arising from the left side His and its adjacent region: a subset of upper septal idiopathic left ventricular tachycardia. Europace 2023;25:1000–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Jhuo SJ, Lin YJ, Chen SA. Different directions of conduction of fascicular potential and ventricular potential during left posterior fascicular ventricular tachycardia ablation: what is the mechanism? Europace 2017;19:118. [DOI] [PubMed] [Google Scholar]
- 85. Zhou G, Lu X, Nie Z, Chen S, Wei Y, Cai Let al. . QRS complex axis deviation changing in catheter ablation of left fascicular ventricular tachycardia. Europace 2020;22:1688–96. [DOI] [PubMed] [Google Scholar]
- 86. Crawford T, Mueller G, Good E, Jongnarangsin K, Chugh A, Pelosi F Jret al. . Ventricular arrhythmias originating from papillary muscles in the right ventricle. Heart Rhythm 2010;7:725–30. [DOI] [PubMed] [Google Scholar]
- 87. Lee J, Adeola O, Garan H, Stevenson WG, Yarmohammadi H. Electrocardiographic recognition of benign and malignant right ventricular arrhythmias. Europace 2021;23:1338–49. [DOI] [PubMed] [Google Scholar]
- 88. Rivera S, de la Paz Ricapito M, Espinoza J, Belardi D, Albina G, Giniger Aet al. . Cryoablation for ventricular arrhythmias arising from the papillary muscles of the left ventricle guided by intracardiac echocardiography and image integration. JACC Clin Electrophysiol 2015;1:509–16. [DOI] [PubMed] [Google Scholar]
- 89. Zhong L, Lee YH, Huang XM, Asirvatham SJ, Shen WK, Friedman PAet al. . Relative efficacy of catheter ablation vs antiarrhythmic drugs in treating premature ventricular contractions: a single-center retrospective study. Heart Rhythm 2014;11:187–93. [DOI] [PubMed] [Google Scholar]
- 90. Mohanty S, Burkhardt JD, Di Biase L, Mohanty P, Shetty SS, Gianni Cet al. . Best ablation strategy in patients with premature ventricular contractions with multiple morphology: a single-centre experience. Europace 2023;25, euad038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Picano E, Vañó E, Rehani MM, Mont L, Bodi V, Bar Oet al. . The appropriate and justified use of medical radiation in cardiovascular imaging. A position document of the ESC Associations of Cardiovascular Imaging, Percutaneous Cardiovascular Interventions and Electrophysiology. Eur Heart J 2014;35:665–72. [DOI] [PubMed] [Google Scholar]
- 92. Seewöster T, Löbe S, Hilbert S, Bollmann A, Sommer P, Lindemann Fet al. . Cardiovascular magnetic resonance imaging in patients with cardiac implantable electronic devices: best practice and real-world experience. Europace 2019;21:1220–8. [DOI] [PubMed] [Google Scholar]
- 93. Hilbert S, Weber A, Nehrke K, Börnert P, Schnackenburg B, Oebel Set al. . Artefact-free late gadolinium enhancement imaging in patients with implanted cardiac devices using a modified broadband sequence: current strategies and results from a real-world patient cohort. Europace 2018;20:801–7. [DOI] [PubMed] [Google Scholar]
- 94. Romero J, Cerrud-Rodriguez RC, Di Biase L, Diaz JC, Alviz I, Grupposo Vet al. . Combined endocardial–epicardial versus endocardial catheter ablation alone for ventricular tachycardia in structural heart disease: a systematic review and meta-analysis. JACC Clin Electrophysiol 2019;5:13–24. [DOI] [PubMed] [Google Scholar]
- 95. Wijnmaalen AP, van der Geest RJ, van Taxis CF vH, Siebelink HM, Kroft LJ, Bax JJet al. . Head-to-head comparison of contrast-enhanced magnetic resonance imaging and electroanatomical voltage mapping to assess post-infarct scar characteristics in patients with ventricular tachycardias: real-time image integration and reversed registration. Eur Heart J 2011;32:104–14. [DOI] [PubMed] [Google Scholar]
- 96. van Huls van Taxis CF, Wijnmaalen AP, Piers SR, van der Geest RJ, Schalij MJ, Zeppenfeld K. Real-time integration of MDCT-derived coronary anatomy and epicardial fat: impact on epicardial electroanatomic mapping and ablation for ventricular arrhythmias. JACC Cardiovasc imaging 2013;6:42–52. [DOI] [PubMed] [Google Scholar]
- 97. Torri F, Czimbalmos C, Bertagnolli L, Oebel S, Bollmann A, Paetsch Iet al. . Agreement between gadolinium-enhanced cardiac magnetic resonance and electro-anatomical maps in patients with non-ischaemic dilated cardiomyopathy and ventricular arrhythmias. Europace 2019;21:1392–9. [DOI] [PubMed] [Google Scholar]
- 98. Andreu D, Penela D, Acosta J, Fernández-Armenta J, Perea RJ, Soto-Iglesias Det al. . Cardiac magnetic resonance-aided scar dechanneling: influence on acute and long-term outcomes. Heart Rhythm 2017;14:1121–8. [DOI] [PubMed] [Google Scholar]
- 99. Hennig A, Salel M, Sacher F, Camaioni C, Sridi S, Denis Aet al. . High-resolution three-dimensional late gadolinium-enhanced cardiac magnetic resonance imaging to identify the underlying substrate of ventricular arrhythmia. Europace 2018;20:f179–f91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Andreu D, Ortiz-Pérez JT, Boussy T, Fernández-Armenta J, de Caralt TM, Perea RJet al. . Usefulness of contrast-enhanced cardiac magnetic resonance in identifying the ventricular arrhythmia substrate and the approach needed for ablation. Eur Heart J 2014;35:1316–26. [DOI] [PubMed] [Google Scholar]
- 101. Paetsch I, Sommer P, Jahnke C, Hilbert S, Loebe S, Schoene Ket al. . Clinical workflow and applicability of electrophysiological cardiovascular magnetic resonance-guided radiofrequency ablation of isthmus-dependent atrial flutter. Eur Heart J Cardiovasc Imaging 2019;20:147–56. [DOI] [PubMed] [Google Scholar]
- 102. Mukherjee RK, Costa CM, Neji R, Harrison JL, Sim I, Williams SEet al. . Evaluation of a real-time magnetic resonance imaging-guided electrophysiology system for structural and electrophysiological ventricular tachycardia substrate assessment. Europace 2019;21:1432–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Al-Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis ABet al. . 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: executive summary: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the Heart Rhythm Society. Circulation 2018;138:e210–71. Erratum in: Circulation. 2018; 138:e415-e418. PMID: 29084733. [DOI] [PubMed] [Google Scholar]
- 104. Tung R, Vaseghi M, Frankel DS, Vergara P, Di Biase L, Nagashima Ket al. . Freedom from recurrent ventricular tachycardia after catheter ablation is associated with improved survival in patients with structural heart disease: an international VT ablation center collaborative group study. Heart Rhythm 2015;12:1997–2007.Epub 2015 May 30. PMID: 26031376; PMCID: PMC4549209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Frankel DS, Mountantonakis SE, Robinson MR, Zado ES, Callans DJ, Marchlinski FE. Ventricular tachycardia ablation remains treatment of last resort in structural heart disease: argument for earlier intervention. J Cardiovasc Electrophysiol 2011;22:1123–8. [DOI] [PubMed] [Google Scholar]
- 106. Dinov B, Arya A, Bertagnolli L, Schirripa V, Schoene K, Sommer Pet al. . Early referral for ablation of scar-related ventricular tachycardia is associated with improved acute and long-term outcomes: results from the heart center of Leipzig ventricular tachycardia registry. Circ Arrhythm Electrophysiol 2014;7:1144–51. [DOI] [PubMed] [Google Scholar]
- 107. Reddy VY, Reynolds MR, Neuzil P, Richardson AW, Taborsky M, Jongnarangsin Ket al. . Prophylactic catheter ablation for the prevention of defibrillator therapy. N Engl J Med 2007;357:2657–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Kuck KH, Schaumann A, Eckardt L, Willems S, Ventura R, Delacrétaz Eet al. . Catheter ablation of stable ventricular tachycardia before defibrillator implantation in patients with coronary heart disease (VTACH): a multicentre randomised controlled trial. Lancet 2010;375:31–40.PMID: 20109864. [DOI] [PubMed] [Google Scholar]
- 109. Kuck KH, Schaumann A, Eckardt L, Willems S, Ventura R, Delacrétaz Eet al. . Impact of substrate modification by catheter ablation on implantable cardioverter-defibrillator interventions in patients with unstable ventricular arrhythmias and coronary artery disease: results from the multicenter randomized controlled SMS (substrate modification study). Circ Arrhythm Electrophysiol 2017;10:e004422. PMID: 28292751. [DOI] [PubMed] [Google Scholar]
- 110. Willems S, Tilz RR, Steven D, Kääb S, Wegscheider K, Gellér Let al. . Preventive or deferred ablation of ventricular tachycardia in patients with ischemic cardiomyopathy and implantable defibrillator (BERLIN VT): a multicenter randomized trial. Circulation 2020;141:1057–67. [DOI] [PubMed] [Google Scholar]
- 111. Tung R, Xue Y, Chen M, Jiang C, Shatz DY, Besser SAet al. . First-line catheter ablation of monomorphic ventricular tachycardia in cardiomyopathy concurrent with defibrillator implantation: the PAUSE-SCD randomized trial. Circulation 2022;145:1839–49.Epub 2022 May 4. PMID: 35507499. [DOI] [PubMed] [Google Scholar]
- 112. Arenal Á, Ávila P, Jiménez-Candil J, Tercedor L, Calvo D, Arribas Fet al. . Substrate ablation vs antiarrhythmic drug therapy for symptomatic ventricular tachycardia. J Am Coll Cardiol 2022;79:1441–53. PMID: 35422240. [DOI] [PubMed] [Google Scholar]
- 113. Della Bella P, Baratto F, Vergara P, Bertocchi P, Santamaria M, Notarstefano Pet al. . Does timing of ventricular tachycardia ablation affect prognosis in patients with an implantable cardioverter defibrillator? Results from the multicenter randomized PARTITA trial. Circulation 2022;145:1829–38. Epub 2022 Apr 3. PMID: 35369700. [DOI] [PubMed] [Google Scholar]
- 114. Moss AJ, Schuger C, Beck CA, Brown MW, Cannom DS, Daubert JPet al. . Reduction in inappropriate therapy and mortality through ICD programming. N Engl J Med 2012;367:2275–83. Epub 2012 Nov 6. PMID: 23131066. [DOI] [PubMed] [Google Scholar]