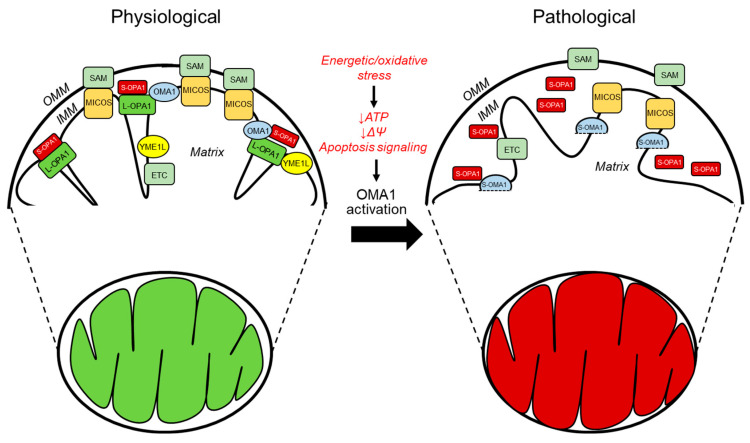
Figure 2.
The primary regulators of mitochondrial cristae organization in both physiological and pathological conditions. The diagram depicts the structural arrangement of mitochondrial membranes, in which the cristae arise from the inward folding of the inner membrane towards the matrix. The MICOS complex, which is responsible for organizing mitochondrial contact sites and cristae, is situated at the CJs. It comprises seven subunits, namely, MIC10, MIC13, MIC19, MIC25, MIC26, MIC27, and MIC60. The stabilization of the CJs and establishing contacts between the IMM and OMM requires MICOS to be involved. The convergence of MICOS and the SAM complex results in the formation of a more extensive entity known as the mitochondrial intermembrane space bridging complex, which encompasses the intermembrane space. The protein OPA1 is highly localized at the CJs and its presence is crucial for preserving the proper dimensions of these structures. This necessitates the interplay between the membrane-bound long (L-) variants and the soluble short (S-) variants of OPA1. Under physiological (denoted in green) and pathological (denoted in red) conditions, the IMM proteases YME1L and OMA1 regulate OPA1. The protein OMA1 is responsible for cleaving all isoforms of OPA1 at the S1 site, whereas YME1L is responsible for cleaving OPA1 at the S2 site but only for the subset of OPA1 that contains the splice variant. Under physiological conditions, YME1L modifies the L-OPA1 to S-OPA1 ratio of the heterooligomeric complexes by cleaving a subset of the L-OPA1 isoforms at the S2 cleavage site. Under normal circumstances, OMA1 exhibits minimal activity; however, it is known to become activated in response to pathological conditions. OMA1 cleaves all L-OPA1 isoforms at the S1 cleavage site, releasing S-OPA1 from the IMM and OMA1 self-cleavage resulting in S-OMA1. Additionally, under stress conditions, the SAM-Mic19-Mic-60 axis can be disrupted through the cleavage of Mic19 by OMA1, resulting in the separation of the SAM and MICOS. Overall, the activation of OMA1 under pathological circumstances leads to impaired mitochondrial function.