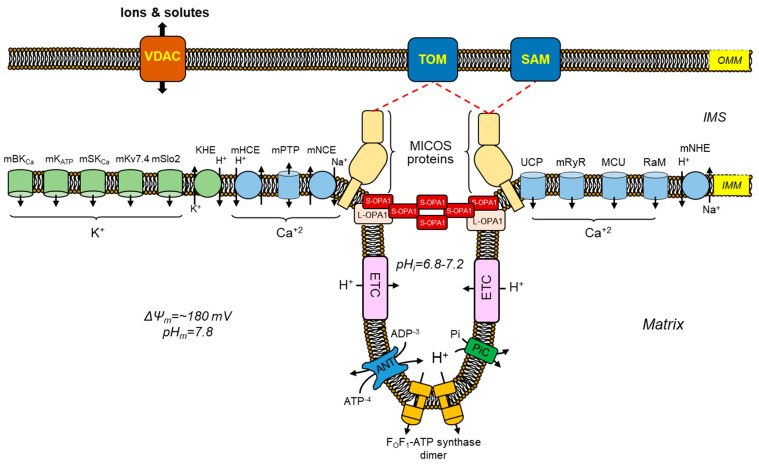
Figure 3.
Mechanisms involved in maintaining ion homeostasis, structural maintenance, and matrix volume within the mitochondria. A graphical representation that illustrates the primary influx and efflux channels of Ca2+ and K+, which are responsible for regulating the volume, as well as the structural constituents that govern the morphology of cardiac mitochondria. The primary mechanisms responsible for Ca2+ influx are the MCU, RaM, mRyR, and uncoupling proteins 2 and 3 (UCP2/3). The maintenance of Ca2+ and ion homeostasis in the matrix is a crucial aspect, wherein the Ca2+ efflux mechanisms such as mitochondrial mNCE, mHCE, and mPTP play a significant role. The transportation of K+ holds equal significance for the metabolism and functioning of mitochondria. Alterations in the concentration of K+ exhibit a direct correlation with variations in the volume of the mitochondrial matrix. The mechanisms responsible for K+ influx comprise the mBKCa, mKATP, mSKCa, mKv7.4, and mSlo2. Conversely, the K+ efflux mechanisms are restricted to the KHE. The function of OPA1 is the fusion of IMM and its regulatory role in cristae morphogenesis. In the heart, five isoforms of OPA1 exist that can be characterized as either L-OPA1 to S-OPA1. The various isoforms of OPA1 have demonstrated the capacity to oligomerize and uphold stringent CJs. A protein complex known as MICOS facilitates preserving a stable state in mitochondrial cristae junctions. In addition to its membrane bending capabilities, MICOS exhibits interactions with proteins situated in the OMM, including the translocase of the outer membrane (TOM) complex, the sorting and assembly machinery (SAM), and the voltage-dependent anion channel (VDAC) protein. Furthermore, the mitochondrial Na+/H+ exchanger (mNHE) has been implicated in the maintenance of ion homeostasis within mitochondria. Also, the invaginations of CJs are generated by dimeric FOF1-ATP synthase, where CJ structures serve as the site for ETC complexes, ANT, and phosphate carrier (PiC), which utilize the pH gradient to facilitate the process of ATP synthesis.