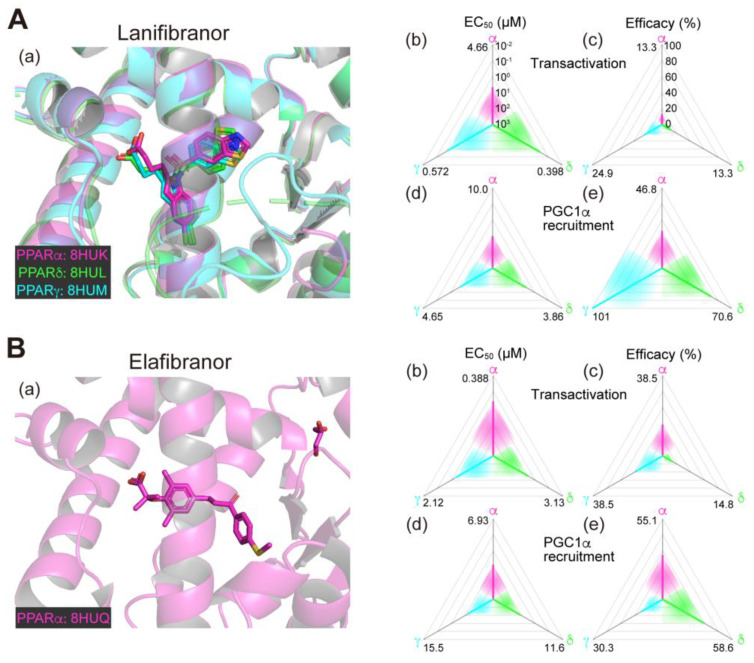
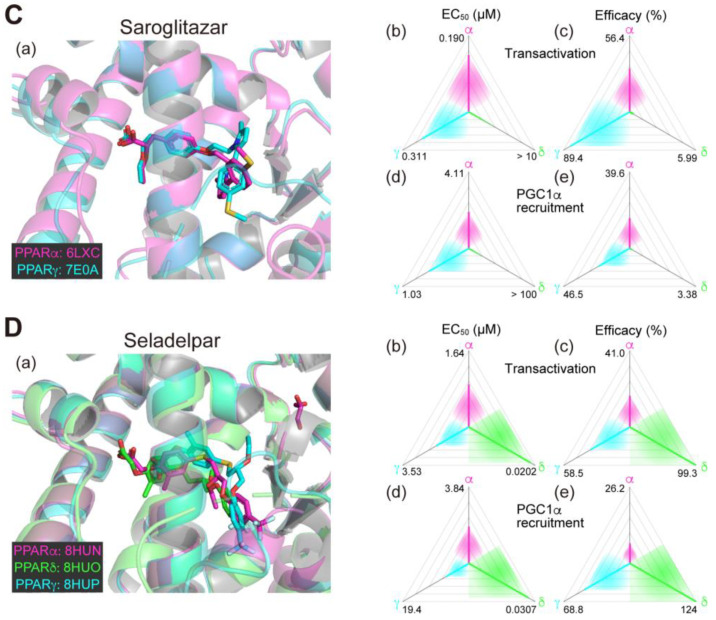
Figure 1.
Binding modes in the PPAR cocrystal structures and the potencies/efficacies in transactivation and PGC1α recruitment activity of lanifibranor (A), elafibranor (B), saroglitazar (C), and seladelpar (D) against PPARα/δ/γ. (a) Merged magnified views of ligands bound to the PPARα (magenta)/δ (green)/γ (light blue)-ligand binding domains revealed by X-ray diffraction analyses of cocrystals; Protein Data Bank (PDB) IDs are shown. PPARδ/γ–elafibranor and PPARδ–saroglitazar cocrystals were not obtained. (b–e) Potencies as EC50 values (µM) (b,d), and efficacies as % of the maximal responses triggered by the PPARα/δ/γ-selective full agonists (GW7647, GW501516, and GW1929, respectively) (c,e) in GAL4-based transactivation assay in Cos-7 cells (b,c) and time-resolved fluorescence energy transfer (TR-FRET)-based PGC1α coactivator recruitment assay (d,e). In each ternary plot, the degrees of potency and efficacy are shown by the axes from the triangle center to the three vertices (PPARα in magenta, PPARδ in green, and PPARγ in light blue) on logarithmic (b,d) and linear scales (c,e), respectively. All structural and functional data were published by our group [11,12,13,14,20].