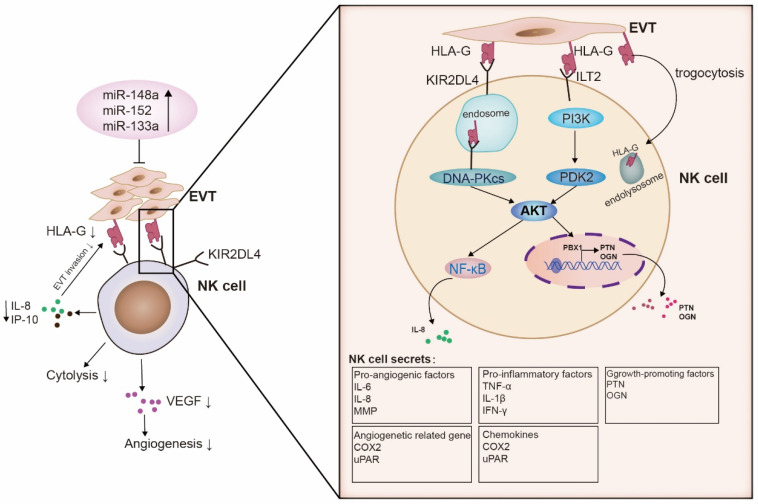
Figure 2.
Schematic of the molecular interaction between HLA-G and decidual NK cells at the maternal–fetal interface. Several microRNAs (miR-133a, miR-152, and miR-148a) bind to the 3′ UTR of HLA-G, resulting in the downregulation of HLA-G expression in trophoblast cells. When miRNAs are bound to HLA-G, they influence the secretion ability of dNK cells upon interaction with KIR2DL4. KIR2DL4, in turn, interacts with DNA-PKcs and initiates a signaling pathway involving serine/threonine kinases DNA-PKcs and Akt, as well as NF-κB. This pathway leads to the expression of the senescence-associated secretory phenotype (SASP), which includes pro-inflammatory factors like TNF-α, IL-1β, and IFN-γ, as well as pro-angiogenic factors such as IL-6 and IL-8. In addition, ILT2 activates the PI3K-AKT signaling pathway, resulting in the secretion of the growth-promoting factors pleiotrophin (PTN) and osteoglycin (OGN), which facilitate early fetal growth. HLA-G-incorporated NK cells possess lower toxicity. NK cells that acquire HLA-G from EVT via trogocytosis have reduced cytotoxicity. They are important for maintaining the immune tolerance of the maternal–fetal interface and the viral immunity.