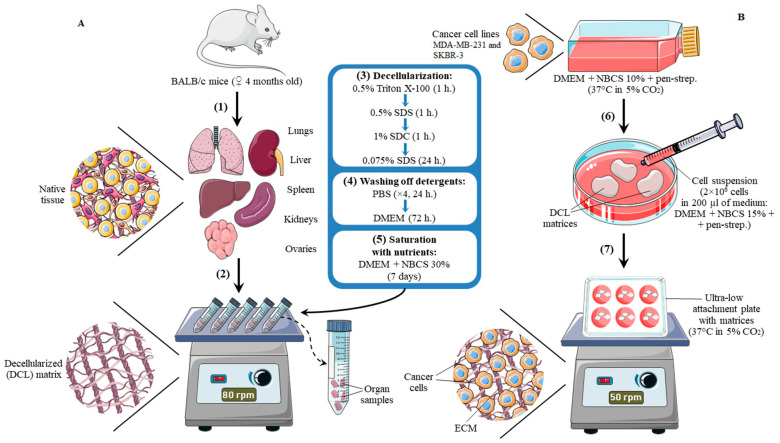
Figure 1.
Simplified scheme of decellularization (A) and recellularization (B) procedures. The liver, kidneys, ovaries, spleen, and lungs were collected (1) from mice of BALB/c line in sterile conditions. The collected organs were washed in sterile distilled water. Fat adhesions were removed from each organ, after which the organs were dissected into 0.3 × 0.3 cm pieces and placed in 50 mL tubes (2). For decellularization (3), the samples were subsequently incubated in Triton X-100, sodium dodecyl sulfate (SDS) solution, sodium deoxycholate (SDC) solution, and again in SDS solution. At each stage of the protocol, the tubes with samples were fixed on an orbital shaker with a rotation speed of 80 rpm. After decellularization, the samples were washed off the detergents (4) with PBS and DMEM media and saturated with nutrients (5) in DMEM media with 30% newborn calf serum (NBCS) for 7 days with a change of medium every 72 h. For recellularization, each preconditioned matrix was placed in an individual well of a 6-well ultra-low attachment plate and repopulated by injecting 300,000 cells in 500 μL of complete growth media (6). The cell suspension was distributed inside the matrix by several injections via an insulin syringe; 5 mL of DMEM with 15% serum was added to each well of the plate, and the matrices were incubated at 37 °C in an atmosphere of 5% CO2 for 7 days (7). On days 3 and 5, the medium bathing the matrix was collected and the cell pellet was re-injected into matrices.