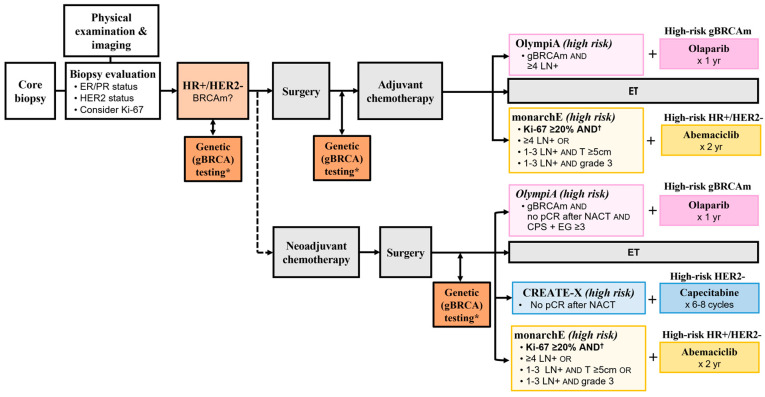
Figure 2.
Treatment pathways for high-risk HR+/HER2- early breast cancer. “High-risk disease” criteria for the OlympiA trial (pink), CREATE-X trial (blue), and CADTH recommendation for abemaciclib (yellow) are overlayed. In HR+/HER2- disease, surgery followed by adjuvant chemotherapy is commonly pursued within the current standards of care; the neoadjuvant chemotherapy pathway is less common (dotted line). ER, estrogen receptor; ET, endocrine therapy; BRCAm, BRCA mutation; CADTH, Canadian Agency for Drugs and Technologies in Health; gBRCA, germline BRCA; gBRCAm, germline BRCA-mutated; HR, hormone receptor; LN, lymph node; NACT, neoadjuvant chemotherapy; pCR, pathological complete response; PR, progesterone receptor. * If not done earlier. † For abemaciclib: The CADTH reimbursement recommendation requires Ki-67 ≥20% and specific clinicopathological features per monarchE Cohort 1. Cohort 2 did not receive a funding recommendation [56].