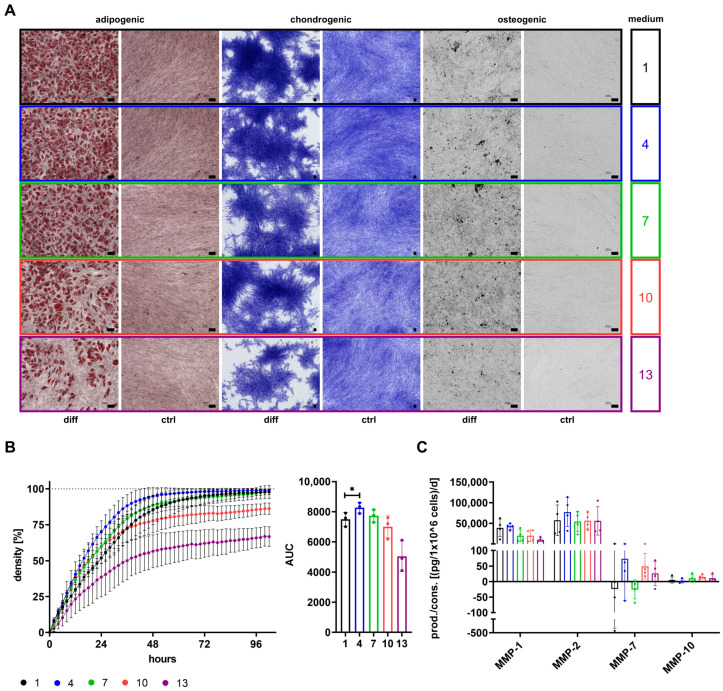
Figure 7.
Differentiation and migration potential of MSCs grown in media 1, 4, 7, 10, and 13. MSCs were expanded in media 1 (100% αMEM+8%PL; black), 4 (95% αMEM+8%PL + 5% StemMACSTM; blue), 7 (50% αMEM+8%PL + 50% StemMACSTM; green), 10 (5% αMEM+8%PL + 95% StemMACSTM; red) and 13 (100% StemMACSTM; violet) and analyzed for their differentiation potential and migratory capacity. (A) MSCs were differentiated into cells of adipogenic, chondrogenic, and osteogenic lineages by culture in specific differentiation media (diff). Control cells were expanded in αMEM+20%FCS (ctrl). Cells of adipogenic differentiation were stained by Oil Red O and hematoxylin. Methylene blue staining was performed to detect chondrogenic differentiation. Activity of alkaline phosphatase was visualized by 5-bromo-4-chloro-3-indolylphosphate (BCIP)/nitroblue tetrazolium (NBT) substrate to detect osteogenic differentiation. Pictures of cells were taken by an inverted phase contrast microscope with 4 times (chondrogenic) and 10 times (adipogenic and osteogenic) magnification, respectively. Black scale bars indicate 100 µm. (B) Migratory potential of cells was investigated by a scratch wound assay. For this, cells were grown in media 1, 4, 7, 10, and 13 in a 96-well plate until confluence of cell cultures was reached. A scratch wound area was created into the cell layer and migration of cells was analyzed for 96 h. Relative cell density was identified by IncuCyte® S3 Live-Cell Analysis system. The area under the curve (AUC) was determined for observed analyses curves of wound density over time. (C) The secretion of MMP-1, MMP-2, MMP-7, and MMP-10 was analyzed for cells grown in media 1, 4, 7, 10, and 13. Data are presented as mean ± SD and N = 2 (A) or N ≥ 3 (B,C) independent experiments were performed. Statistically significant differences are depicted as follows: *: p < 0.05. Representative images were chosen for differentiation assays.