Figure 3.
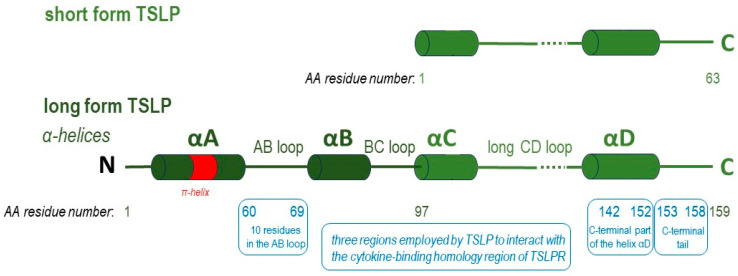
Long form TSLP (lfTSLP), consisting of 159 amino acids (AA), has four α-helices (αA, αB, αC, αD) linked by a BC loop and two longer AB and CD loop regions. Short form TSLP is 63 AA residues in length and covers the C-terminal half of human TSLP (residues 97–159) (adapted after [15]). The regions employed by TSLP to interact with the cytokine-binding homology region of TSLPR are marked in boxes in blue. Tezepelumab targets TSLP at the C-terminal region of helix D and AB loop region. The plasticity of the π-helical turn in αA has an critical functional role in the priming of TSLP by TSLPR to enable high-affinity binding by IL-7Rα; therefore, IL-7Rα cannot be recruited to the TSLP:tezepelumab complex.