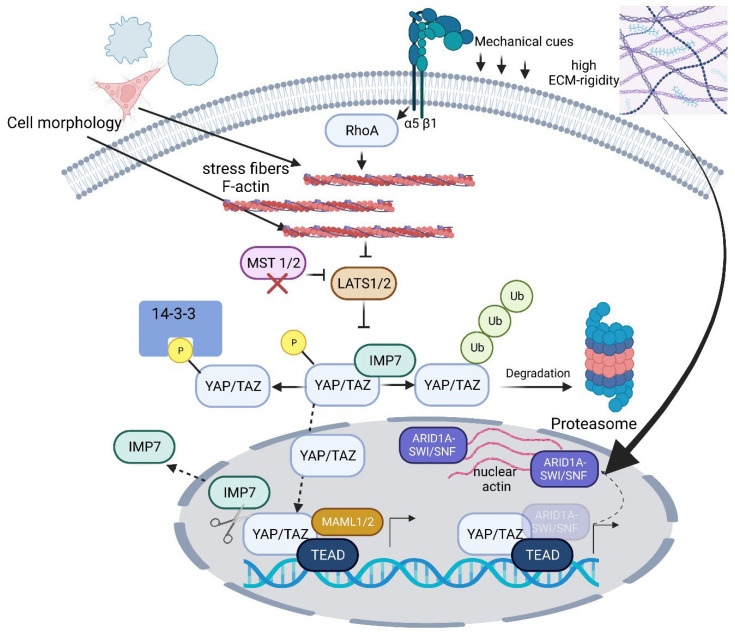
Figure 1.
Schematic overview of YAP/TAZ signaling in mechanotransduction. YAP/TAZ illustrated as nuclear relays of mechanical signals exerted by the ECM rigidity and cell shape, which requires Rho GTPase (RhoA) activity and tension of the actomyosin cytoskeleton. YAP/TAZ molecules are inhibited through phosphorylation by the Hippo-innate large tumor suppressor kinase 1/2 (LATS1/2). In the case of YAP, this occurs through the LATS1/2-dependent phosphorylation of YAP at serine residue 127. Following this phosphorylation, YAP interacts with the 14-3-3 protein and is thus retained in the cytoplasm and subsequently ubiquitinated and degraded. For the co-transcription function, YAP shuttles back and forth between the nucleus and the cytoplasm. Therefore, it must enter the nucleus after being released from the cytoplasmic 14-3-3 protein. Its entry is mediated by nuclear transport receptors (NTRs), and YAP specifically binds to Importin 7 (IMP7) as an NTR. YAP/IMP7 interaction requires the inactivation of MST1/2, which, in turn, activates the YAP-inhibiting LATS kinase. The permanent function of YAP as a co-transcriptional activator requires its interaction with MAML1/2, acting as transcriptional co-activators by forming a trimeric complex with YAP/TAZ and TEAD to induce the gene transcription of YAP/TAZ-specific genes. The YAP/TAZ complex formation dynamics are regulated by a protein complex called ARID1A-SWI/SNSF. This complex binds to YAP/TAZ, preventing interaction with TEAD. Moreover, in response to high ECM rigidity, nuclear actin increasingly polymerizes and binds to the ARID1A-SWI/SNF complex. This interaction between the complex and nuclear actin facilitates the progressive release of YAP/TAZ from the AR-ID1A-SWI/SNF complex and, therefore, allows for YAP/TAZ interaction with TEAD to initiate the transcription of target genes. The schematic was created with BioRender.com.