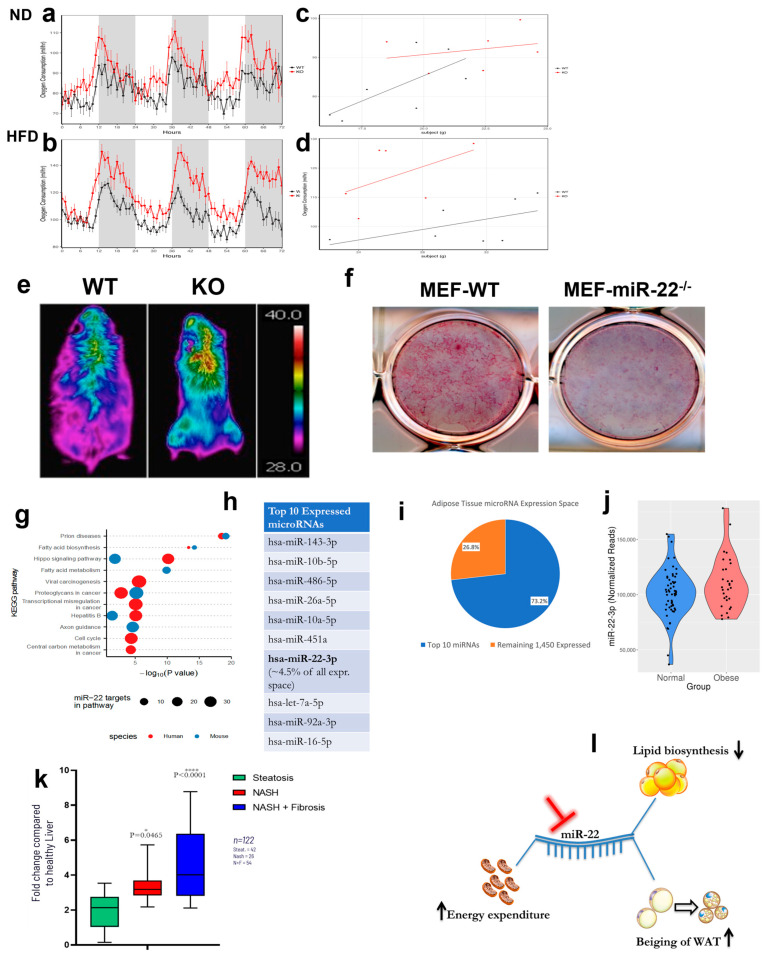
Figure 2.
Genetic ablation of miR-22 prevents diet-induced obesity through metabolic rewiring. (a) Metabolic cage analysis shows that there is no difference in VO2 consumption between WT and miR-22 KO mice when fed a normal diet (n = 7). (b) Metabolic cage analysis shows that after 8 weeks on an HFD, KO mice have significantly higher VO2 consumption than WT mice despite lack of weight gain on the HFD (n = 7). (c) Regression analysis between VO2 and body mass from WT and KO mice fed with ND (n = 7). (d) Regression analysis between VO2 and body mass from WT and KO mice fed with an HFD for 8 weeks (n = 7). (e) Representative thermal images of WT and miR-22−/− mice after 8 weeks on an HFD. The miR-22−/− mice are leaner than WT littermates, and they are characterized by a higher body temperature in the interscapular area, where BAT is located (n = 5 per cohort). (f) MEF cells from WT and miR-22−/− mice were isolated and cultured in presence of adipose differentiation media for 8 days and then stained with Oil-Red-O. Staining of WT cells is much more intense than miR-22-deficient cells, implying that genetic ablation of miR-22 impairs adipocyte differentiation, making miR-22 null cells less prone to differentiate into adipocytes. (g) miRNA pathway analysis performed in humans and mice identifies pathways with overrepresentation of miR-22 target genes in TarBase and miR-22 targets in Reactome pathways. We used Diana-microT to predict miR-22 targets in humans and mice. Subsequently, we identified the number of predicted miR-22 targets annotated to different Reactome pathways. miR-22 is predicted to target genes across almost all major pathways in Reactome. (h) The 10 most highly expressed miRNAs in 185 human abdominal subcutaneous adipose tissue samples from the METSIM study [23562819]. (i) Pie chart depicting the number and percent (%) of next-generation sequencing reads assigned to the 10 most highly expressed miRNAs and the remaining 1450 miRNAs identified as expressed (>=1 sequencing read assigned) in 185 human abdominal subcutaneous adipose tissue samples from the METSIM study [23562819]. (j) miR-22 expression in human abdominal subcutaneous adipose tissue samples of individuals with BMI > 30 (n = 30) and BMI < 25 (n = 56) from the METSIM study [23562819]. Obese individuals exhibit increased miR-22 expression (p-value: 0.0078; q-value: 0.0373). (k) miR-22 level in different cohort of human patients diagnosticated with steatosis but not fibrosis, or F1-F2 fibrosis stage, or F3-F4 fibrosis stage. miR-22 level directly correlate with disease progression in human patients. (l) Proposed model of how miR-22 genetic inhibition impacts obesity and steatosis through different pathways that converge on the same metabolic advantage and protective effects.