Abstract
Objective:
To systematically review and compare the overall (OS) and disease-free (DFS) survival after hepatic resections for hepatocellular carcinoma (HCC) of patients with nonalcoholic fatty liver disease (NAFLD) versus other risk factors.
Background:
Different clinical and tumor characteristics are associated with HCC in the setting of NAFLD in comparison to other risk factors. It is still unclear whether these differences impact patient survival after radical hepatectomies.
Methods:
Randomized controlled trials and observational studies published in the English literature between July 1980 and June 2020 were searched using multiple databases. Patients’ baseline characteristics and the hazard ratios (HRs) of the OS and DFS were extracted and meta-analyses were performed.
Results:
Fifteen retrospective cohort studies with a total of 7226 patients were included. Among them, 1412 patients (19.5%) had NAFLD and 5814 (80.4%) had other risk factors (eg, viral hepatitis B or C, alcoholic cirrhosis, or cryptogenic cirrhosis). Summary statistics showed that patients with NAFLD had better DFS (HR = 0.81; 95% CI: 0.70–0.94; P = 0.006) and OS (HR = 0.78; 95% CI: 0.67–0.90; P = 0.001) than the control group. Subgroups analyses also indicated that the OS favored NAFLD patients versus patients with viral hepatitis B or C (HR = 0.80; 95% CI: 0.67–0.96; P = 0.017) or alcoholic and cryptogenic cirrhosis (HR = 0.68; 95% CI: 0.47–1.0; P = 0.05).
Conclusion:
After hepatic resections for HCC, NAFLD patients have better DFS and OS than patients with other risk factors. Subgroup analysis and meta-regression suggested that the survival advantage of NAFLD patients was more pronounced in studies published after 2015 and from Asian centers.
Keywords: meta-analysis, meta-regression, systematic review, hepatoma, hepatocellular carcinoma, hepatic resection, nonalcoholic steatohepatitis, nonalcoholic fatty liver disease, overall survival, disease-free survival, viral hepatitis B, viral hepatitis C, cryptogenic cirrhosis, alcoholic cirrhosis
Mini Abstract: This meta-analysis examines the disease-free survival (DFS) and the overall survival (OS) of NAFLD patients undergoing hepatic resection for hepatocellular carcinoma compared to patients with other risk factors. The summary statistics from data extracted from fifteen observational studies suggest that NAFLD patients have more favorable DFS and OS than the control group.
Supplemental Digital Content is available in the text.
INTRODUCTION
Hepatocellular carcinoma (HCC) is the most common primary tumor of the liver and the fifth most frequent malignancy worldwide.1 Surgery, in the form of liver transplantation or hepatic resection, provides the longest survival.2–4 Liver transplantation has the best cure rate,5–7 however, resection is the most common surgical therapy as liver transplantation is limited by the number of donors and, in some countries, by insufficient resources.5–7
Worldwide, the highest incidence of HCC is found in South-East Asia and sub-Saharan Africa while the lowest is found in North America and Northern Europe.8 These geographical differences are due to distinct risk factors. While hepatitis B virus (HBV) is the primary cause of HCC in Asia and Africa,9,10 viral hepatitis C (HCV), alcoholic cirrhosis, and nonalcoholic fatty liver disease (NAFLD) are the most common risk factors in Europe and the United States.11
Over the last few decades, the incidence of HCC from viral hepatitis has decreased due to the introduction of vaccination programs for hepatitis B12 and antiviral drugs for HCV.13 The opposite trend, however, is seen for NAFLD14 not only in Western countries where obesity is widespread but also in Africa, the Middle East, and Asia.15–18 The reasons why the prevalence of NAFLD is on the rise globally are still unclear, yet there is growing evidence that there are oncological and clinical differences between HCC in the settings of NAFLD versus other risk factors. For instance, patients with NAFLD have an upregulation of STAT-1 and STAT-3, 2 proteins linked to hepatocarcinogenesis in the absence of cirrhosis.19,20 Also, compared to tumors from other risk factors, HCC in the settings of NAFLD has different gene regulatory networks21 and nucleotide polymorphisms.22 Whether these differences impact the survival after hepatic resections with curative intent remains uncertain. While some studies have suggested that patients with NAFLD have worse outcomes,23–26 others have reported opposite finding27–37 even though patients with NAFLD are usually older and with more comorbidities.19,38,39
To better understand the outcomes of NAFLD patients compared to patients with other risk factors, we performed a systematic review and a meta-analysis to test the null hypothesis that there are no significant differences in the overall survival (OS) and disease-free survival (DFS) between the 2 groups after radical resections for HCC. Our secondary aim was to assess if the results of the meta-analyses were dependent on specific effect modifiers such as the presence of cirrhosis, etiology of liver disease, geography, and year of publication.
MATERIAL AND METHODS
This study was structured using the Population, Intervention, Comparison, Outcome (PICO) template. Adult patients (18 years or older) represented the study population irrespective of ethnicity or risk factors for HCC. For intervention, we selected hepatic resections performed with curative intent. Patients who had synchronous locoregional therapies such as radiofrequency or microwave ablations at the time of hepatic resections were excluded. The surgical techniques (eg, open surgery, laparoscopic surgery, or hybrid surgery), and the extent of resection (eg, lobectomy, segmentectomy, trisegmentectomy, or nonanatomical resections) were not factors included in the analysis. All comparisons were made between patients with NAFLD versus other risk factors for HCC. The primary outcome measure was the OS, while the secondary outcome was the DFS.
The PRISMA (preferred reporting items for systematic reviews and meta-analyses)40 and MOOSE (meta-analysis of observational studies in epidemiology)41 guidelines were used for the conduct and reporting of this study. (Appendices S1 and S2, Supplementary Table 1, http://links.lww.com/AOSO/A37, and Supplementary Table 2, http://links.lww.com/AOSO/A37.)
Data Sources and Searches
A systematic review of the English literature was completed according to a protocol that outlined the primary and secondary objectives, the inclusion and exclusion criteria of the studies, the type of data to be collected, and the statistical analysis to be performed. The protocol was registered with the PROSPERO database at www.crd.york.ac.uk/prospero, registration number CRD 207395.
The PubMED/MEDLINE, EMBASE, Scopus, Google Scholar, the Cochrane Library, Web of Science, ClinicalTrials.gov, International Clinical Trials Registry Platform, Networked Digital Library of Theses and Dissertations, and Dissertations and Theses Global databases were searched to identify studies published between July 1, 1980, and June 30, 2020. The initial date restriction for the search of the literature was based on the fact that NAFLD was described for the first time in July 1980.42
Since all studies on NAFLD patients occurred after 1980, we searched only databases in the English language as it had already become the international language of choice for scientific publications.
Two health science librarians (C.W. and M.L.K.) developed and executed the search strategy. Searches were limited to studies on humans, published in the English language using terms and keywords. The subject explosion was used for scientific terms.
Terms and keywords used for the search referred to HCC, hepatoma, liver neoplasm, nonalcoholic steatohepatitis (NASH), NAFLD, surgery, and survival. More specifically, the terms and keywords to identify suitable studies included the following terminology and Boolean logic: (hepatocellular carcinoma OR liver neoplasm OR hepatoma) AND (non-fatty liver disease OR nonalcoholic steatohepatitis) AND (surgery OR resection OR lobectomy OR segmentectomy OR wedge resection OR anatomical resection OR non-anatomical resection) AND (overall survival or disease-free survival or outcomes).
Electronic searches were also complemented by hand-searching of the reference list of identified articles and bibliographies of relevant books and review articles. For studies reporting the outcomes of several treatment groups, each group was assessed for eligibility.
Study Selection
The titles and the abstracts of the retrieved articles were stored using the web-based software DistillerSR, (Evidence Partners, Ottawa, Canada). Two independent investigators (M.M. and P.B.S) screened the titles to assess if they were relevant for this study. When the content of the titles was not sufficiently informative, the respective abstracts and articles were fully appraised.
Randomized controlled trials, quasi-randomized controlled studies, case-control, and cohort studies were eligible. Studies were excluded if they were duplicate publications, if hepatic resections were performed on patients with mixed hepatocellular-cholangiocarcinomas or fibrolamellar carcinomas, if patients had previous liver transplants, or if they underwent hepatic resections with synchronous combined locoregional therapies such as ablations.
When the same institution or the same group of investigators published data originating from cohorts of patients at different time intervals, we included only the articles with the largest population or the articles with the highest quality.
Studies that did not report sufficient data on patient survival after hepatic resections were also excluded.
Data Extraction
All variables used for this meta-analysis were abstracted by 2 independent investigators (M.M. and P.B.S.). Pertinent data were recorded on standardized preprinted forms developed during the writing of the research protocol. If discrepancies of the data collected by the 2 reviewers (M.M. and P.B.S.) were identified, a third investigator (C.K.) was consulted for reconciliation.
The variables collected for each study were the name of the primary author, the year of publication, the country where patients were enrolled, the type of study design categorized into randomized controlled study, quasi-randomized controlled study and observational study, the year of initiation of the study, the year of completion of the study, the total number of patients enrolled, the number of patients with NAFLD and the number of patients with other types of liver disease, the type of chronic liver disease affecting patients in the control group, the mean or median age of the study population, the mean or median serum level of alpha-fetoprotein (AFP), the percentage of patients with cirrhosis, and the median size or the pTNM stage of the tumors.
The diagnosis of cirrhosis was based on the pathological analysis of the surgical specimens reported in the original studies included in this meta-analysis.
Assessment of Bias
Two independent investigators (P.B.S. and M.M.) assessed the quality of the articles. In the case of discrepancies, a third researcher (C.K.) was consulted. Since no randomized controlled studies were identified by the literature search, the Newcastle-Ottawa Assessment Scale (NOS)20 was used to measure the quality of the articles.
The NOS is a validated instrument43,44 designed to assess the quality of nonrandomized studies for meta-analyses.20 It contains 8 multiple-choice questions that are related to the selection of patients, their comparability, and their outcomes (Supplementary Table 3, http://links.lww.com/AOSO/A37).
In the NOS, 9 stars represent the highest level of quality. Studies with 7 or more stars are categorized as good quality, 5 to 6 stars indicate fair quality, and 4 or fewer stars indicate poor quality. Stars are allocated as follows: up to 4 stars for patient selection, up to 2 stars for comparability, and up to 3 stars for exposure/outcomes.
Publication bias was evaluated using funnel plots quantified by Egger regression analysis.45
Endpoints
The primary and secondary aims of this study were the OS and the DFS of NAFLD patients versus patients with other risk factors for HCC. Within the control group, risk factors for HCC were categorized into viral hepatitis (B, C, or other), alcoholic cirrhosis, cryptogenic cirrhosis, and unspecified. The OS was defined as the time between the date of hepatic resection and the date of death from any cause. The DFS was defined as the time between the date of hepatic resection and the date of diagnosis of recurrent HCC by radiological tests, rising levels of serum tumor markers, physical examination, or the combination of all 3 modalities.
Summary Statistics
The hazard ratios (HRs) with corresponding 95% confidence intervals (95% CIs) were used as measures of treatment effects. When studies reported both the crude and the adjusted HRs (aHR), the aHR was preferred.
For studies where the HR was not available, an indirect estimate of the HRs with respective 95% CIs was obtained using Parmar’s methodology.46 Parmar’s methodology allows the calculation of the HR using data obtained from Kaplan–Meier survival curves or using the actuarial percentages of surviving patients at specific time intervals. DigitizeIt, a software designed to extract data from scientific graphics, was used to compute the percentages of surviving patients for the indirect calculation of the HR. The percentages of patients who survived at different time intervals were subsequently entered in an open-access Microsoft Excel spreadsheet calculator46 developed to compute the HR and 95% CI by the cooperation of the Meta-Analysis Group, MRC Clinical Trials Unit, London, UK, School of Public Health, Sydney, Australia, and MRC Clinical Trial Unit, London, UK.47 The HR calculator used for this study is publicly accessible at the Uniform Resource Locator: http://www.biomedcentral.com/content/supplementary/1745-6215-8-16-S1.xls.
Data Synthesis
The random-effect model48 was used for all the summary statistics and meta-regressions.49 For meta-regressions, the year of publication and the proportion of NAFLD patients affected by cirrhosis were used as independent variables (potential effect modifiers). The heterogeneity among studies was assessed using the I2 statistics.50 I2 is an estimate of the proportion of variability due to between-study heterogeneity rather than by sampling errors.51 I2 values of 25%, 50%, and 75% were considered thresholds of a low, moderate, and high degree of heterogeneity, respectively.50
All statistical analyses were performed using Comprehensive Meta-Analysis (CMA) Version 3 (Biostat Inc.) and 2-tailed P values of 0.05 were considered statistically significant.
RESULTS
The initial electronic search yielded 179,908 articles. During the first screening, 145,603 articles were excluded based on the content of their titles. The remaining 33,980 studies underwent a second screening, and 10,723 articles were excluded because of duplicate publications and 23,092 articles because they were not relevant for our study.
A total of 165 articles were fully appraised and their references were hand-searched. No additional studies were found by hand search. After reviewing the full content of 165 articles that passed the second screening, we excluded 150 as they did not meet all the inclusion criteria. Inter-rater reliability for the exclusion and inclusion of the studies used for this meta-analysis was 100%, and the final number of articles that were included was 15. The flowchart summarizing the different stages of the systematic review of the literature, the number of retrieved studies, and the reasons for their exclusion are reported in Figure 1.
FIGURE 1.
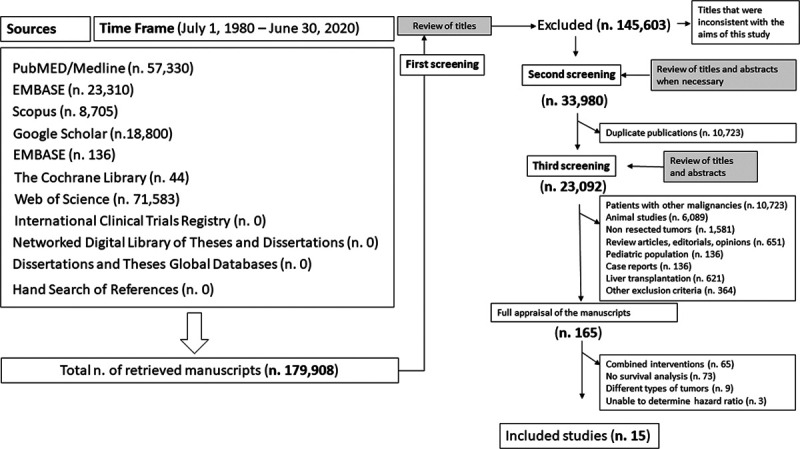
Flowchart of the systematic review of the literature throughout different phases. The literature search was performed from July 1, 1980, to June 30, 2020. PubMed/Medline, EMBASE, Scopus, Google Scholar, EMBASE, The Cochrane Library, Web of Science, International Clinical Trials Registry, Network Digital Library of Theses and Dissertations, Dissertations and Theses Global Databases were searched in addition to hand search of references of pertinent articles or books. Initially, a total of 179,908 titles were retrieved. During the first screening, 145,603 articles were excluded as their titles revealed that the respective articles did not fulfill the inclusion criteria. During the second screening, a total of 10,723 articles were excluded because found to be duplicate publications. By the third screening, 23,092 studies were excluded, and the remaining 165 articles were fully appraised. A total of 150 studies were excluded as they did not report postoperative patient survival (n = 73), they included patients undergoing multiple interventions (n = 65), they included patients with tumors that were not hepatocellular carcinomas (n = 9), or the hazard ratios were not reported or there was not enough data to estimate the hazard ratios (n = 3). Overall, the total number of studies that were included in this meta-analysis was 15.
Study Characteristics
The main characteristics of the articles used for this meta-analysis are reported in Supplementary Table 4, http://links.lww.com/AOSO/A37. Patients were enrolled over 25 years (from 1990 to 2015), 11 studies (73.3%) were from Asia, 3 (20%) were from Europe, and 1 (6.6%) was from the United States. All studies were retrospective cohort studies of good quality. The summary of the quality metrics of the studies based on the NOS is summarized in Appendix S4, Supplementary Table 5, http://links.lww.com/AOSO/A37. Interrater reliability for the quality of the studies was 86%. The funnel plot of the log of the HR (x-axis) over the standard error (y-axis)45 had a symmetric distribution suggesting a low-risk of publication bias (see Appendix S5, Supplementary Figure 1S, http://links.lww.com/AOSO/A37).
Patient and Tumor Characteristics
Baseline characteristics of the study population are reported in Supplementary Table 6, http://links.lww.com/AOSO/A37. The total number of patients was 7226 with a median/mean age ranging between 51 and 72 years, 1412 (19.5%) patients had NAFLD, and 5814 (80.4%) patients had other risk factors for HCC. The minimum follow-up was 24 months, while the longest was 87 months. The prevalence of cirrhosis ranged between 10.5% and 75% for patients with NAFLD, and between 19.1% and 93% for patients within the control group.
Disease-Free Survival
Data on the DFS was available in 12 studies. The 5-year DFS ranged from 24.4% to 66% for NAFLD patients compared to 17.4% to 46.9% for patients within the control group (see Appendix S6, Supplementary Table 7, http://links.lww.com/AOSO/A37). The summary statistics from the data obtained from all 12 studies favored NAFLD patients (pooled HR = 0.81; 95% CI = 0.70–0.94; P = 0.006; I2 = 55.1%) (Fig. 2A). Rosenthal’s52 Fail-Safe N analysis estimated that 87 additional studies would be necessary to nullify the difference in DFS between NAFLD patients and the control group.
FIGURE 2.
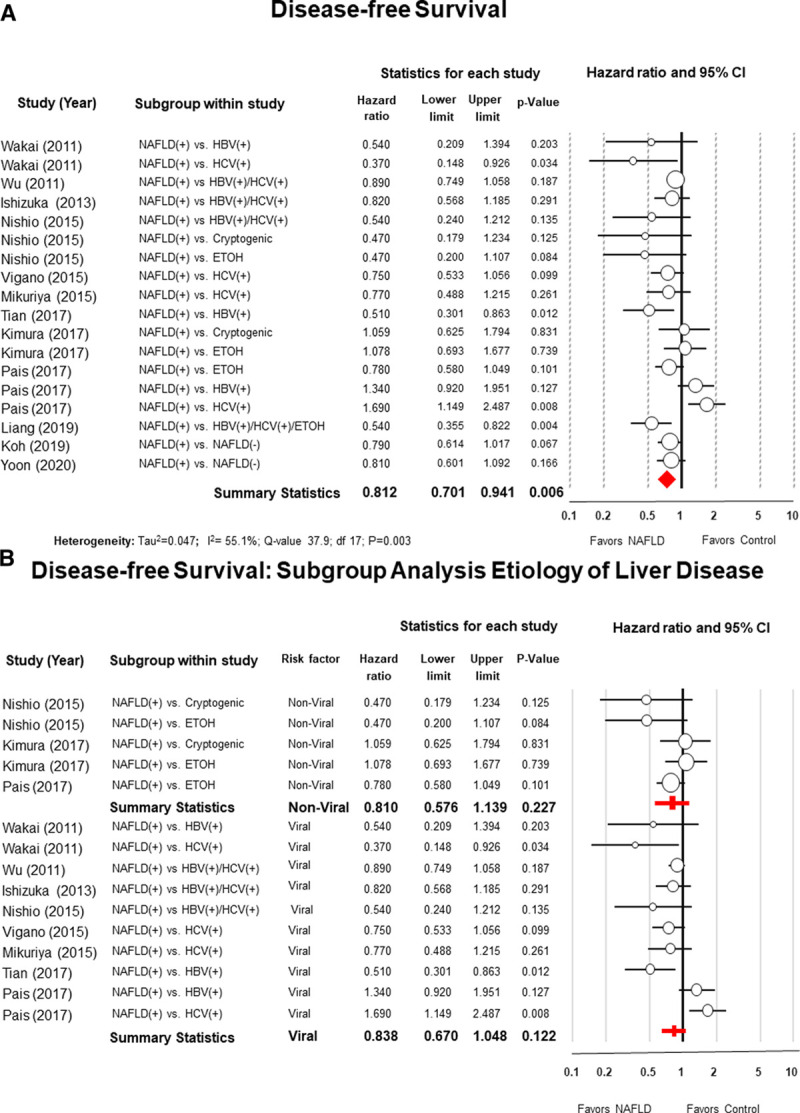
Panel A, Forest plot of meta-analysis with random effect model of the disease-free survival (DFS) of patients with nonalcoholic fatty liver disease (NAFLD) after hepatic resection for hepatocellular carcinoma compared to patients with other risk factors. Panel B, Subgroup analysis comparing NAFLD patients with patients with non-viral risk factors (alcoholic or cryptogenic cirrhosis) and with viral hepatitis (B or C).
Subgroup analyses showed no differences in DFS between NAFLD patients and patients with viral hepatis (B or C) (HR = 0.83; 95% CI: 0.67–1.04; P = 0.12; I2 = 66.7%) or patients with other types of nonviral liver diseases (alcoholic or cryptogenic cirrhosis) (HR = 0.81; 95% CI: 0.57–1.13; P = 0.22; I2 = 25.3%) (Fig. 2B).
Overall Survival
Data on the OS was available in 14 studies. For NAFLD patients, the 5-year OS ranged from 28.1% to 91.1% compared to 21.2% to 79.2% for the control group (see Appendix S7, Supplementary Table 8, http://links.lww.com/AOSO/A37). The summary statistics from the data obtained from all 14 studies favored NAFLD patients (pooled HR = 0.78; 95% CI: 0.67–0.90; P = 0.001; I2 = 87.9%) (Fig. 3A).
FIGURE 3.
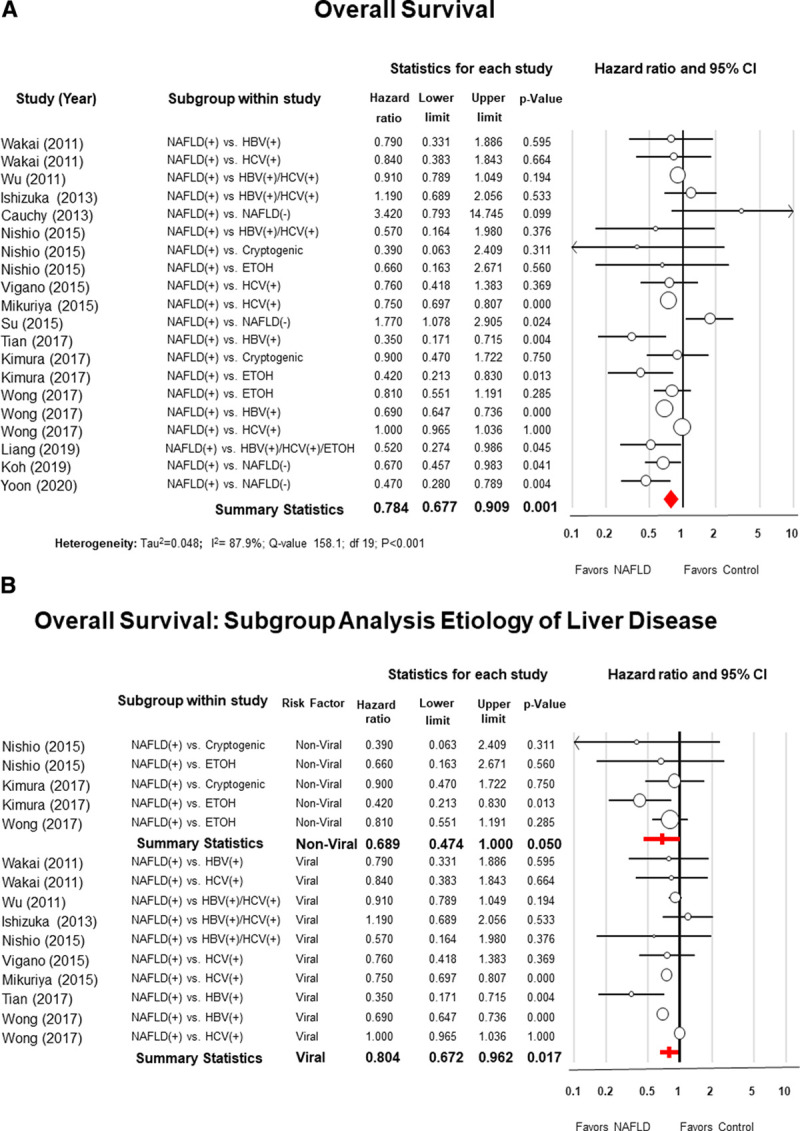
Panel A, Forest plot of meta-analysis with random effect model of the overall survival (OS) of patients with nonalcoholic fatty liver disease (NAFLD) after hepatic resection for hepatocellular carcinoma compared to patients with other risk factors. Panel B, Subgroup analysis comparing NAFLD patients with patients with nonviral risk factors (alcoholic or cryptogenic cirrhosis) and in the setting of viral hepatitis (B or C).
Rosenthal’s (52) Fail-Safe N analysis estimated that a total of 227 additional studies would be necessary to nullify the difference of the OS between NAFLD patients and the control group.
Subgroup analysis showed that patients with NAFLD had a better OS in comparison to patients with viral hepatis (B or C) (HR = 0.80; 95% CI: 0.67–0.96; P = 0.01; I2 = 93.1%) or nonviral liver diseases (alcoholic or cryptogenic cirrhosis) (HR = 0.68; 95% CI: 0.47–0.99; P = 0.05; I2 = 12.3%) (Fig. 3B).
Meta-Regressions and Subgroup Analyses
We hypothesized that improvement in patient selection, surgical techniques, and perioperative care occurring over the study period might have contributed to the heterogeneity of the data of this meta-analysis. To test our hypothesis, we performed a meta-regression and sensitivity analysis using the year of publication as an independent variable. The meta-regression showed that there was a statistically significant relationship between the log of the HR and the year of publication indicating that the difference in the OS between NAFLD and the control group increased over time (Fig. 4A).
FIGURE 4.
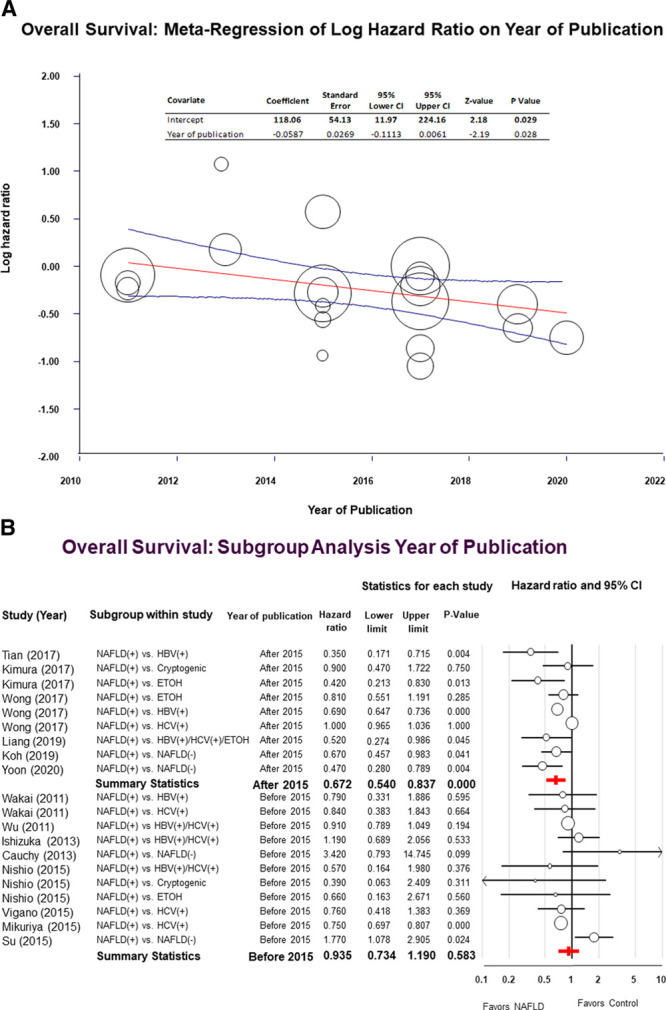
Panel A, Univariate meta-regression showing the effects of the year of publication on the log of the hazard ratio (HR). Compared to the control group, the overall survival (OS) advantage of patients with nonalcoholic fatty liver disease (NAFLD) increased over time (P = 0.02). Each circle represents a study, and the area of each circle is proportional to the relative weight of the respective study. The central line represents the fitted regression line with respective 95% confidence intervals. Panel B, Forest plot for the sensitivity analysis assessing the effects of the year of publication on the OS of NAFLD patients compared to patients with other risk factors. Studies published after 2015 are reported on the upper part of the forest plot while studies published before 2015 are reported on the lower part of the forest plot.
Subgroup analysis was performed using the year 2015 as discriminant to separate studies into 2 groups with a balanced distribution of the number of articles. For studies published before 2015, we found no significant difference in the OS between NAFLD patients and the control group (HR = 0.93; 95% CI: 0.73–1.19; P = 0.58; I2 = 56.1%). On the other hand, when studies published after 2015 were included, the pooled HR for the OS favored NAFLD patients (HR = 0.67; 95% CI: 0.54–0.83; P = 0.001; I2 = 93.4%) (Fig. 4B).
Cirrhosis is a well-known factor affecting postoperative survival after hepatic resections. Therefore, a meta-regression analysis was performed using the prevalence of cirrhosis as a continuous independent variable. Contrary to our hypothesis, we found no statistically significant associations between the HR for DFS and OS and the prevalence of cirrhosis. The summary of the results of meta-regression analysis for DFS and OS are reported in Appendix S8, Supplementary Table 9, http://links.lww.com/AOSO/A37.
Subgroup analyses were also performed to determine if the results were influenced by the geographical location where the studies had been performed. The HR for DFS favored NAFLD in Asia (HR = 0.73; 95% CI: 0.62–0.87; P < 0.001; I2 25.2) while there was no significant difference between the 2 groups for studies performed in Western countries (HR = 1.04; 95% CI: 0.80–1.35; P = 0.74; I2 79.7) (Fig. 5A).
Figure 5.
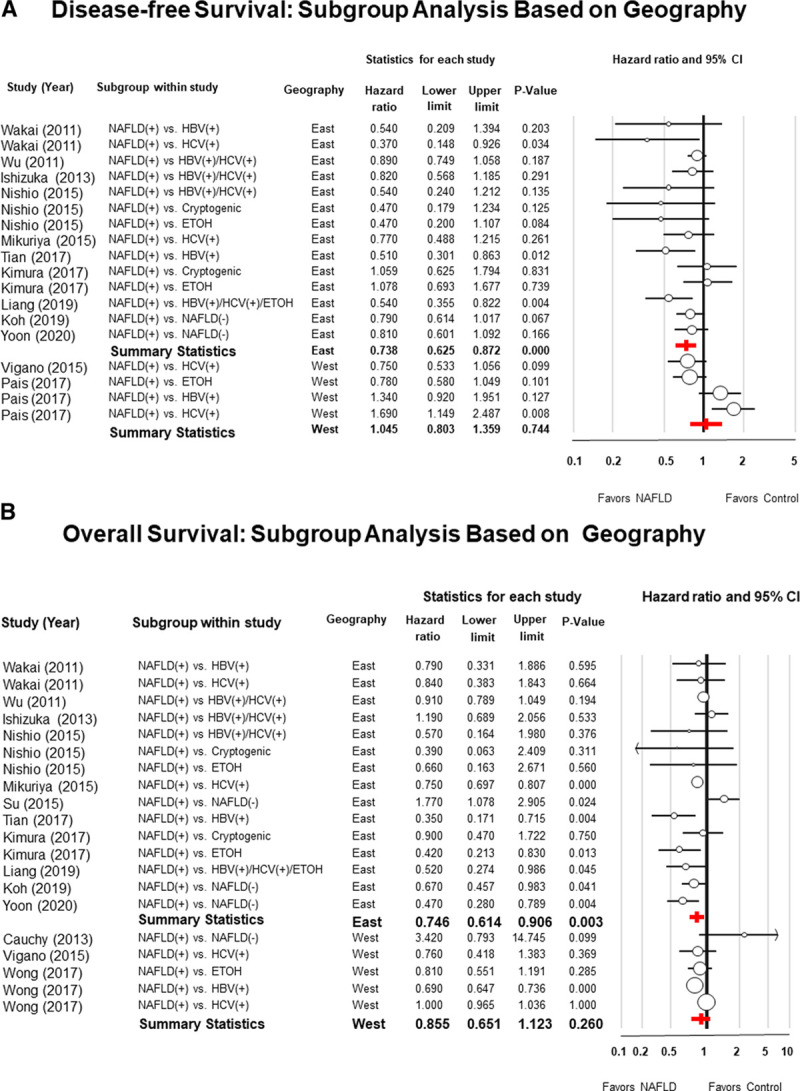
Panel A, Forest plots of meta-analysis with random effect model of the disease-free survival (DFS), and Panel B, overall survival (OS), of patients with nonalcoholic fatty liver disease (NAFLD) after hepatic resection for hepatocellular carcinoma compared to patients with other risk factors. Subgroup analysis was performed based on the geographical area where patients were enrolled.
Similarly, the HR for OS favored NAFLD in Asia (HR = 0.74; 95% CI: 0.61–0.90; P = 0.003; I2 = 57.9), while there was no significant difference between the 2 groups in studies performed in Europe or the United States (HR = 0.85; 95% CI: 0.65–1.12; P = 0.26; I2 = 96.0) (Fig. 5B).
DISCUSSION
Four decades have passed since the term NAFLD was introduced by Ludwig et al42 to describe the histological findings consistent with abnormal deposits of fat in the hepatocytes of patients without a history of alcohol abuse. Since then, NAFLD has become one of the most prevalent liver diseases and a leading cause of HCC worldwide.
The epidemic of NAFLD remains a topic of intense research; however, several important aspects of this condition remain unclear. From the surgical point of view, one of the unanswered questions is whether the survival of NAFLD patients undergoing hepatic resection for HCC is comparable to the survival of patients with other types of chronic liver diseases. While several groups have reported that NAFLD patients have better outcomes,28,30 others have found opposite results.53,54
To address this controversial issue, we performed a systematic review and a meta-analysis using data from 15 observational studies that compared the survival of patients treated with radical hepatic resections for HCC in the setting of NAFLD versus patients with viral or nonviral diseases. Since our primary aim was to characterize the effects of surgery, we did not include studies where patients underwent combined treatments such as hepatic resections and simultaneous ablation therapies. To the best of our knowledge, this is the first meta-analysis to test the null hypothesis that there are no differences in the DFS and OS between these 2 groups of patients.
The main finding of this study was that the HR for the DFS and OS of NALFD patients was significantly lower than the HR of patients with other risk factors. Sensitivity analysis also indicated that the DFS and the OS were superior for NAFLD patients irrespective of the type of liver disease affecting the control group.
The data used for this meta-analysis was extracted from observational studies with very high scores of the NOS indicating good quality.44 Although all the articles had similar study designs, inclusion and exclusion criteria, indications for surgery, interventions, and duration of follow-ups, we found that they had significant variability as indicated by the high values of the I2 statistics. Several reasons might have contributed to this heterogeneity. Patients had comparable tumors and equivalent treatments, but their enrollment occurred over 25 years and in centers located in different continents. Over time, patient selection, operative techniques, and perioperative therapies had improved, and we suspect that the combination of all these factors might have contributed to the degree of heterogeneity that we observed in this meta-analysis.
Also, most studies (73%) were from Asia, 3 from Europe, and only 1 from the United States. Therefore, it is conceivable that distinct patient and tumor characteristics, as well as differences in the clinical management of patients, were responsible for the overall heterogeneity. To better understand the impact of these factors, we performed subgroup and meta-regression analyses to investigate how the pooled HRs changed based on the geography, the year of publication, and the prevalence of cirrhosis in the 2 groups. Since the quality of the studies was homogeneous, sensitivity analysis based on the quality of the studies was not done.
Geography
Asian patients represented the majority of the population. Oncological and clinical variations between Eastern and Western populations have been well described by other investigators.55 Differences involve not only the predisposing factors for HCC56 but also the genomic profile of the tumors,57,58 the clinical characteristics of the patients,59,60 and how they are managed by their healthcare providers.61
From the surgical point of view, Asian surgeons tend to have a more aggressive approach than surgeons practicing in Western countries.4,62 For example, in Asia, surgical resections are often performed even in the presence of multifocal HCCs and in patients with cirrhosis or portal hypertension as long as the liver function and the future liver remnant volume are satisfactory.63–65
From the point of view of the tumor characteristics, Asian patients are affected by HCCs at a younger age, are more often affected by tumors with poor tumor cell differentiation, higher p53 expression,66 and have a better prognosis than non-Asian populations.67
Sensitivity analysis showed that the DFS favored NAFLD patients in Asia (HR = 0.73; 95% CI: 0.62–0.87; P < 0.001), while there was no significant difference between the 2 groups in studies from Europe and North America (HR = 1.045; 95% CI: 0.80–1.35; P = 0.74).
Similar findings were observed for the OS that favored Asian patients with NAFLD (HR = 0.746; 95% CI: 0.61–0.90; P = 0.003), while there was no survival difference between the 2 groups when only studies from Europe and North America were included (HR = 0.85; 95% CI: 0.65–1.12; P = 0.26).
The lack of significant differences between the 2 groups in Western countries might be due to an insufficient number of patients as well as the possibility that NAFLD patients from Europe and the United States had different clinical or tumor characteristics than Asian patients. Unfortunately, we were unable to further explore this aspect due to the lack of more granular data from the original studies.
Year of Publication
The OS advantage of NAFLD patients was more pronounced for studies performed in more recent years. This effect was appreciated by the presence of a negative linear correlation between the year of publication and the log of the HR of the OS of NAFLD patients. When studies published before 2015 were included, the difference in the OS between the 2 groups was not significant (HR = 0.93; 95% CI: 0.73–1.19; P = 0.58). On the other hand, analysis of the data from studies published after 2015 showed a significant OS advantage for NAFLD patients with an HR of 0.67 (95% CI: 0.51–0.83; P < 0.001).
A possible reason for the negative correlation between the HR and the year of publication was that over the years, surgeons and other healthcare providers might have gained more experience in how to manage and select patients with NASH who are more often affected by cardiopulmonary and other metabolic conditions that increase their risk of postoperative mortality.68,69
Prevalence of Cirrhosis
Opposite to patients with other risk factors, 10% to 75% of NAFLD patients develop HCCs in the absence of cirrhosis.69–71 Since cirrhosis is a major risk factor for perioperative mortality and long-term survival,39,72,73 we performed a meta-regression to test if cirrhosis was an important effect modifier. Contrary to our expectations, cirrhosis did not modify the results of this meta-analysis. We suspect that this negative finding was probably due to the similar prevalence of cirrhosis within the two groups. Consequently, our study was underpowered to detect differences in patient survival based on the presence or absence of cirrhosis and further investigations will be necessary to address this issue.
Strengths and Limitations of This Study
One of the strengths of this meta-analysis is the high quality of the included studies and the rigorous methodology used for the extraction of the data and the subgroup and meta-regression analyses performed to assess the impact of predefined effect modifiers.
The protocol used to conduct this meta-analysis was developed and registered before starting the systematic review of the literature, the extraction of the data, and the statistical analyses. Therefore, misleading post hoc analyses were avoided as they were not part of the published protocol.74 Another strength was the assistance of 2 experienced health-science librarians who independently performed the systematic search of the literature that decreased the risk of missing relevant evidence. The systematic review of the literature identified a very large number of studies that were screened by 2 independent investigators to ascertain that all suitable articles were appraised. These efforts led to the inclusion of a large number of patients that enhanced the precision of the summary estimates.
Additional strengths were the use of dual data extraction and dual data entry to avoid the risk of incorrect transcription of the variables of interest.
Despite these strengths, some limitations should be noted. Ideally, individual patient data are preferable to aggregate data for the assessment of interactions. We believed that the likelihood of accessing the original data of studies performed over 25 years was very low; therefore, we did not make any attempt to obtain individual patient data from the primary investigators.
Also, we were unable to identify data from unpublished sources or the gray literature, and some evidence might have been involuntarily overlooked. Another limitation is that all the data used for this meta-analysis were from retrospective studies. Therefore, the risk of biases inherent to the original studies could not be completely adjusted. We also found significant heterogeneity among studies, probably due to differences in patients’ characteristics, tumor factors, and the changes in the clinical management that have inevitably occurred over time. Finally, most studies were from centers located in Asia, and the results of this meta-analysis might not fully apply to patients from non-Asian countries.
CONCLUSIONS
The present meta-analysis represents the most comprehensive review of the literature on the outcomes of patients with NAFLD undergoing radical hepatic resections for HCC.
The results of this study suggest that after radical resections, NAFLD patients have more favorable DFS and OS in comparison to patients with other risk factors for HCC.
The longer survival of NAFLD patients was not contingent on the risk factors affecting the control group. Although these results might be due to the fact that cirrhosis is less common in patients with NAFLD compared to patients with other risk factors, our analysis failed to show that cirrhosis was an important effect modifier. However, further studies will be necessary to confirm this observation.
The current review also demonstrates that most studies on the outcomes of hepatic resections for HCC are from Asia. Therefore, there is the need for more studies from Western centers to better characterize the outcomes of non-Asian patients after hepatic resections for HCC in the settings of NAFLD.
Acknowledgments
M.M. designed the study, extracted the data, assessed the quality of the studies, did the statistical analyses, and wrote the manuscript. C.K. drafted the manuscript, and reconciled discrepancies between reviewers. P.B.-S. did the literature search and screening, extracted the data, assessed the quality of studies, and critically reviewed the manuscript. H.L., S.D., B.E., H.A.H., D.G., A.T., C.H., A.H., and R.B. critically revised the manuscript. C.W. performed the literature search. M.L.K. performed the literature search. S.T. critically reviewed the manuscript. J.B. reviewed the protocol, was the content expert, and critically reviewed the manuscript.
Supplementary Material
Footnotes
Disclosure: The authors state that they have no proprietary interest in the products named in this article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.annalsofsurgery.com).
REFERENCES
- 1.El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011; 365:1118–1127 [DOI] [PubMed] [Google Scholar]
- 2.Kim KH, Choi YK. Long-term survival after resection of hepatocellular carcinoma. Korean J Hepatobiliary Pancreat Surg. 2012; 16:98–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Island ER, Pomposelli J, Pomfret EA, et al. Twenty-year experience with liver transplantation for hepatocellular carcinoma. Arch Surg. 2005; 140:353–358 [DOI] [PubMed] [Google Scholar]
- 4.Bruix J, Sherman M; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011; 53:1020–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang K, Chen R, Gong X, et al. Survival outcomes of liver transplantation versus liver resection among patients with hepatocellular carcinoma: A SEER-based longitudinal study. J Formos Med Assoc. 2019; 118:790–796 [DOI] [PubMed] [Google Scholar]
- 6.Harries L, Schrem H, Stahmeyer JT, et al. High resource utilization in liver transplantation-how strongly differ costs between the care sectors and what are the main cost drivers?: a retrospective study. Transpl Int. 2017; 30:621–637 [DOI] [PubMed] [Google Scholar]
- 7.Kow AWC. Transplantation versus liver resection in patients with hepatocellular carcinoma. Transl Gastroenterol Hepatol. 2019; 4:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012; 142:1264–1273.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang HI, Yeh ML, Wong GL, et al. Real-world effectiveness from the Asia Pacific Rim Liver Consortium for HBV risk score for the prediction of hepatocellular carcinoma in chronic hepatitis B patients treated with oral antiviral therapy. J Infect Dis. 2020; 221:389–399 [DOI] [PubMed] [Google Scholar]
- 10.Knudsen ES, Gopal P, Singal AG. The changing landscape of hepatocellular carcinoma: etiology, genetics, and therapy. Am J Pathol. 2014; 184:574–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghouri YA, Mian I, Rowe JH. Review of hepatocellular carcinoma: Epidemiology, etiology, and carcinogenesis. J Carcinog. 2017; 16:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang XY, Huang JM, Lu XM, et al. Changing risk factors for hepatocellular carcinoma in hyperendemic regions in the era of universal hepatitis B vaccination. Cancer Epidemiol. 2020; 67:101775. [DOI] [PubMed] [Google Scholar]
- 13.Alimohammadi A, Holeksa J, Thiam A, et al. Real-world efficacy of direct-acting antiviral therapy for HCV infection affecting people who inject drugs delivered in a multidisciplinary setting. Open Forum Infect Dis. 2018; 5:ofy120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stroffolini T, Trevisani F, Pinzello G, et al. Changing aetiological factors of hepatocellular carcinoma and their potential impact on the effectiveness of surveillance. Dig Liver Dis. 2011; 43:875–880 [DOI] [PubMed] [Google Scholar]
- 15.Onyekwere CA, Ogbera AO, Balogun BO. Non-alcoholic fatty liver disease and the metabolic syndrome in an urban hospital serving an African community. Ann Hepatol. 2011; 10:119–124 [PubMed] [Google Scholar]
- 16.Das K, Das K, Mukherjee PS, et al. Nonobese population in a developing country has a high prevalence of nonalcoholic fatty liver and significant liver disease. Hepatology. 2010; 51:1593–1602 [DOI] [PubMed] [Google Scholar]
- 17.Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016; 64:73–84 [DOI] [PubMed] [Google Scholar]
- 18.Eguchi Y, Hyogo H, Ono M, et al. ; JSG-NAFLD. Prevalence and associated metabolic factors of nonalcoholic fatty liver disease in the general population from 2009 to 2010 in Japan: a multicenter large retrospective study. J Gastroenterol. 2012; 47:586–595 [DOI] [PubMed] [Google Scholar]
- 19.Grohmann M, Wiede F, Dodd GT, et al. Obesity drives STAT-1-dependent NASH and STAT-3-dependent HCC. Cell. 2018; 175:1289–1306.e20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sanyal A, Poklepovic A, Moyneur E, et al. Population-based risk factors and resource utilization for HCC: US perspective. Curr Med Res Opin. 2010; 26:2183–2191 [DOI] [PubMed] [Google Scholar]
- 21.Pocha C, Xie C. Hepatocellular carcinoma in alcoholic and non-alcoholic fatty liver disease-one of a kind or two different enemies? Transl Gastroenterol Hepatol. 2019; 4:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu YL, Patman GL, Leathart JB, et al. Carriage of the PNPLA3 rs738409 C >G polymorphism confers an increased risk of non-alcoholic fatty liver disease associated hepatocellular carcinoma. J Hepatol. 2014; 61:75–81 [DOI] [PubMed] [Google Scholar]
- 23.Su CW, Chau GY, Hung HH, et al. Impact of steatosis on prognosis of patients with early-stage hepatocellular carcinoma after hepatic resection. Ann Surg Oncol. 2015; 22:2253–2261 [DOI] [PubMed] [Google Scholar]
- 24.Ishizuka M, Kubota K, Kita J, et al. Survival after surgery for hepatocellular carcinoma in relation to presence or absence of viral infection. Am J Surg. 2013; 206:187–193 [DOI] [PubMed] [Google Scholar]
- 25.Cauchy F, Zalinski S, Dokmak S, et al. Surgical treatment of hepatocellular carcinoma associated with the metabolic syndrome. Br J Surg. 2013; 100:113–121 [DOI] [PubMed] [Google Scholar]
- 26.Pais R, Fartoux L, Goumard C, et al. Temporal trends, clinical patterns and outcomes of NAFLD-related HCC in patients undergoing liver resection over a 20-year period. Aliment Pharmacol Ther. 2017; 46:856–863 [DOI] [PubMed] [Google Scholar]
- 27.Yoon JS, Lee HY, Chung SW, et al. Prognostic impact of concurrent nonalcoholic fatty liver disease in patients with chronic hepatitis B-related hepatocellular carcinoma. J Gastroenterol Hepatol. 2020; 35:1960–1968 [DOI] [PubMed] [Google Scholar]
- 28.Wu TH, Yu MC, Chan KM, et al. Prognostic effect of steatosis on hepatocellular carcinoma patients after liver resection. Eur J Surg Oncol. 2011; 37:618–622 [DOI] [PubMed] [Google Scholar]
- 29.Wong CR, Njei B, Nguyen MH, et al. Survival after treatment with curative intent for hepatocellular carcinoma among patients with vs without non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2017; 46:1061–1069 [DOI] [PubMed] [Google Scholar]
- 30.Wakai T, Shirai Y, Sakata J, et al. Surgical outcomes for hepatocellular carcinoma in nonalcoholic fatty liver disease. J Gastrointest Surg. 2011; 15:1450–1458 [DOI] [PubMed] [Google Scholar]
- 31.Viganò L, Conci S, Cescon M, et al. Liver resection for hepatocellular carcinoma in patients with metabolic syndrome: a multicenter matched analysis with HCV-related HCC. J Hepatol. 2015; 63:93–101 [DOI] [PubMed] [Google Scholar]
- 32.Tian Y, Lyu H, He Y, et al. Comparison of hepatectomy for patients with metabolic syndrome-related HCC and HBV-related HCC. J Gastrointest Surg. 2018; 22:615–623 [DOI] [PubMed] [Google Scholar]
- 33.Nishio T, Hatano E, Sakurai T, et al. Impact of hepatic steatosis on disease-free survival in patients with non-B non-C hepatocellular carcinoma undergoing hepatic resection. Ann Surg Oncol. 2015; 22:2226–2234 [DOI] [PubMed] [Google Scholar]
- 34.Mikuriya Y, Tashiro H, Kobayashi T, et al. Clinicopathological features of hepatocellular carcinoma in patients with nonalcoholic fatty liver disease. Langenbecks Arch Surg. 2015; 400:471–476 [DOI] [PubMed] [Google Scholar]
- 35.Liang J, Ariizumi SI, Nakano M, et al. Diabetes mellitus and/or nonalcoholic steatohepatitis-related hepatocellular carcinoma showed favorable surgical outcomes after hepatectomy. Anticancer Res. 2019; 39:5639–5643 [DOI] [PubMed] [Google Scholar]
- 36.Koh YX, Tan HJ, Liew YX, et al. Liver resection for nonalcoholic fatty liver disease-associated hepatocellular carcinoma. J Am Coll Surg. 2019; 229:467–478.e1 [DOI] [PubMed] [Google Scholar]
- 37.Kimura T, Kobayashi A, Tanaka N, et al. Clinicopathological characteristics of non-B non-C hepatocellular carcinoma without past hepatitis B virus infection. Hepatol Res. 2017; 47:405–418 [DOI] [PubMed] [Google Scholar]
- 38.Sookoian S, Rohr C, Salatino A, et al. Genetic variation in long noncoding RNAs and the risk of nonalcoholic fatty liver disease. Oncotarget. 2017; 8:22917–22926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tapper EB, Parikh ND. Mortality due to cirrhosis and liver cancer in the United States, 1999-2016: observational study. BMJ. 2018; 362:k2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moher D, Liberati A, Tetzlaff J, et al. ; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009; 151:264–269, W64 [DOI] [PubMed] [Google Scholar]
- 41.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000; 283:2008–2012 [DOI] [PubMed] [Google Scholar]
- 42.Ludwig J, Viggiano TR, McGill DB, et al. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Proc. 1980; 55:434–438 [PubMed] [Google Scholar]
- 43.Oremus M, Oremus C, Hall GB, et al. ; ECT & Cognition Systematic Review Team. Inter-rater and test-retest reliability of quality assessments by novice student raters using the Jadad and Newcastle-Ottawa Scales. BMJ Open. 2012; 2:e001368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hartling L, Milne A, Hamm MP, et al. Testing the Newcastle Ottawa Scale showed low reliability between individual reviewers. J Clin Epidemiol. 2013; 66:982–993 [DOI] [PubMed] [Google Scholar]
- 45.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997; 315:629–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Parmar MK, Torri V, Steward L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998; 17:2815–2834 [DOI] [PubMed] [Google Scholar]
- 47.Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007; 8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986; 7:177–188 [DOI] [PubMed] [Google Scholar]
- 49.Dieleman JL, Templin T. Random-effects, fixed-effects and the within-between specification for clustered data in observational health studies: a simulation study. PLoS One. 2014; 9:e110257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003; 327:557–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002; 21:1539–1558 [DOI] [PubMed] [Google Scholar]
- 52.Rosenthal R. The “File drawer problem” and tolerance for null results. Phychol Bull. 1979; 86:638–641 [Google Scholar]
- 53.Younossi ZM, Otgonsuren M, Henry L, et al. Association of nonalcoholic fatty liver disease (NAFLD) with hepatocellular carcinoma (HCC) in the United States from 2004 to 2009. Hepatology. 2015; 62:1723–1730 [DOI] [PubMed] [Google Scholar]
- 54.Weinmann A, Alt Y, Koch S, et al. Treatment and survival of non-alcoholic steatohepatitis associated hepatocellular carcinoma. BMC Cancer. 2015; 15:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Choo SP, Tan WL, Goh BKP, et al. Comparison of hepatocellular carcinoma in Eastern versus Western populations. Cancer. 2016; 122:3430–3446 [DOI] [PubMed] [Google Scholar]
- 56.Gomaa AI, Khan SA, Toledano MB, et al. Hepatocellular carcinoma: epidemiology, risk factors and pathogenesis. World J Gastroenterol. 2008; 14:4300–4308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Laurent-Puig P, Zucman-Rossi J. Genetics of hepatocellular tumors. Oncogene. 2006; 25:3778–3786 [DOI] [PubMed] [Google Scholar]
- 58.Zucman-Rossi J, Jeannot E, Nhieu JT, et al. Genotype-phenotype correlation in hepatocellular adenoma: new classification and relationship with HCC. Hepatology. 2006; 43:515–524 [DOI] [PubMed] [Google Scholar]
- 59.Chaturvedi N. Ethnic differences in cardiovascular disease. Heart. 2003; 89:681–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li J, Dong Y, Wu T, et al. Differences between Western and Asian type 2 diabetes patients in the incidence of vascular complications and mortality: a systematic review of randomized controlled trials on lowering blood glucose. J Diabetes. 2016; 8:824–833 [DOI] [PubMed] [Google Scholar]
- 61.Holvoet T, Raevens S, Vandewynckel YP, et al. Systematic review of guidelines for management of intermediate hepatocellular carcinoma using the Appraisal of Guidelines Research and Evaluation II instrument. Dig Liver Dis. 2015; 47:877–883 [DOI] [PubMed] [Google Scholar]
- 62.Arii S, Sata M, Sakamoto M, et al. Management of hepatocellular carcinoma: Report of Consensus Meeting in the 45th Annual Meeting of the Japan Society of Hepatology (2009). Hepatol Res. 2010; 40:667–685 [DOI] [PubMed] [Google Scholar]
- 63.Park JW, Chen M, Colombo M, et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE Study. Liver Int. 2015; 35:2155–2166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ishizawa T, Hasegawa K, Aoki T, et al. Neither multiple tumors nor portal hypertension are surgical contraindications for hepatocellular carcinoma. Gastroenterology. 2008; 134:1908–1916 [DOI] [PubMed] [Google Scholar]
- 65.Torzilli G, Belghiti J, Kokudo N, et al. A snapshot of the effective indications and results of surgery for hepatocellular carcinoma in tertiary referral centers: is it adherent to the EASL/AASLD recommendations?: an observational study of the HCC East-West study group. Ann Surg. 2013; 257:929–937 [DOI] [PubMed] [Google Scholar]
- 66.Song TJ, Fong Y, Cho SJ, et al. Comparison of hepatocellular carcinoma in American and Asian patients by tissue array analysis. J Surg Oncol. 2012; 106:84–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yang JD, Hainaut P, Gores GJ, et al. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. 2019; 16:589–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ertle J, Dechêne A, Sowa JP, et al. Non-alcoholic fatty liver disease progresses to hepatocellular carcinoma in the absence of apparent cirrhosis. Int J Cancer. 2011; 128:2436–2443 [DOI] [PubMed] [Google Scholar]
- 69.Paradis V, Zalinski S, Chelbi E, et al. Hepatocellular carcinomas in patients with metabolic syndrome often develop without significant liver fibrosis: a pathological analysis. Hepatology. 2009; 49:851–859 [DOI] [PubMed] [Google Scholar]
- 70.Yasui K, Hashimoto E, Tokushige K, et al. ; Japan NASH Study Group. Clinical and pathological progression of non-alcoholic steatohepatitis to hepatocellular carcinoma. Hepatol Res. 2012; 42:767–773 [DOI] [PubMed] [Google Scholar]
- 71.Guzman G, Brunt EM, Petrovic LM, et al. Does nonalcoholic fatty liver disease predispose patients to hepatocellular carcinoma in the absence of cirrhosis? Arch Pathol Lab Med. 2008; 132:1761–1766 [DOI] [PubMed] [Google Scholar]
- 72.Grazi GL, Cescon M, Ravaioli M, et al. Liver resection for hepatocellular carcinoma in cirrhotics and noncirrhotics. Evaluation of clinicopathologic features and comparison of risk factors for long-term survival and tumour recurrence in a single centre. Aliment Pharmacol Ther. 2003; 17(suppl 2):119–129 [DOI] [PubMed] [Google Scholar]
- 73.Vilar-Gomez E, Calzadilla-Bertot L, Wai-Sun Wong V, et al. Fibrosis severity as a determinant of cause-specific mortality in patients with advanced nonalcoholic fatty liver disease: a multi-national cohort study. Gastroenterology. 2018; 155:443–457.e17 [DOI] [PubMed] [Google Scholar]
- 74.Walker E, Hernandez AV, Kattan MW. Meta-analysis: its strengths and limitations. Cleve Clin J Med. 2008; 75:431–439 [DOI] [PubMed] [Google Scholar]


