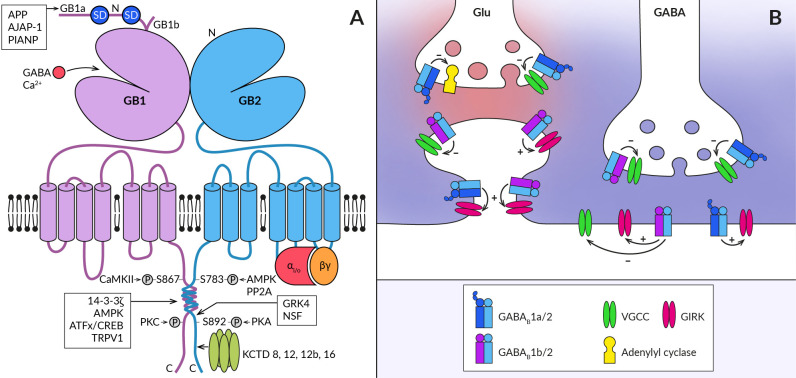
Figure 1.
Schematic representation of the GABABR heterodimer and coupling of its subtypes to effectors at central synapses. (A) The functional GABABR consists of two subunits, GABAB1 (GB1) and GABAB2 (GB2). Both subunits contain large extracellular N-terminal domains (N), seven transmembrane domains connected by three intracellular and three extracellular loops, and an intracellular C-terminus. The N-terminal domain of GABAB1 contains a binding site for the agonist (GABA) and for the endogenous positive allosteric modulator (Ca2+). The two most common splice variants of GABAB1 (GB1a and GB1b) differ in the presence of two sushi domains (SD) at the N-terminus of GABAB1a. Sushi domain binding proteins, the β-amyloid precursor protein (APP), the adherence junction-associated protein 1 (AJAP-1), and the PILRα-associated neural protein (PIANP) form complexes with GABAB1a/2 receptors. The GABAB2 subunit interacts with heterotrimeric G-proteins (αi/o, βγ) and stimulates their activation. At least four phosphorylation sites were identified on GABABR subunits: S867 and a yet unidentified site at the C-terminus on the GABAB1 subunit are phosphorylated by calcium/calmodulin-dependent protein kinase II (CaMKII) and protein kinase C (PKC), respectively; on the GABAB2 subunit, S783 is phosphorylated by 5′AMP-dependent protein kinase (AMPK) and S892 is phosphorylated by protein kinase A and dephosphorylated by protein phosphatase 2A (PP2A). Other GABAB1 interacting proteins include 14-3-3 proteins, the capsaicin receptor TRPV1 and ATF/CREB family transcription factors. By specifically interacting with the latter, GABABR can directly influence gene expression. GABAB2 can further associate with G-protein receptor kinase 4 (GRK4) and N-ethylmaleimide-sensitive factor (NSF), leading to the regulation of GABABR activity. The C-terminus of GABAB2 contains a binding site for auxiliary receptor subunits, proteins of the potassium channel tetramerization domain (KCTD) family. (B) GABABRs are expressed in presynaptic and postsynaptic compartments of both excitatory (Glu) and inhibitory (GABA) synapses (synaptic glutamate and GABA are shown in red and blue, respectively). They associate with effector enzymes and ion channels (adenylyl cyclase, VGCC, GIRK) to regulate neurotransmitter release and neuronal excitability. GABAB1a-containing receptors (GABAB1a/2) are preferentially localized in the presynaptic membrane of both types of synapses and less frequently at postsynaptic sites such as the dendritic shaft or spine neck. In contrast, GABAB1b-containing receptors (GABAB1b/2) prefer postsynaptic sites but are also expressed in inhibitory terminals. GABABRs at inhibitory synapses are activated by synaptic GABA and can mediate slow inhibitory postsynaptic currents. Heteroreceptors at excitatory synapses are activated either tonically by ambient agonist concentration or require GABA spillover from neighboring inhibitory synapses.