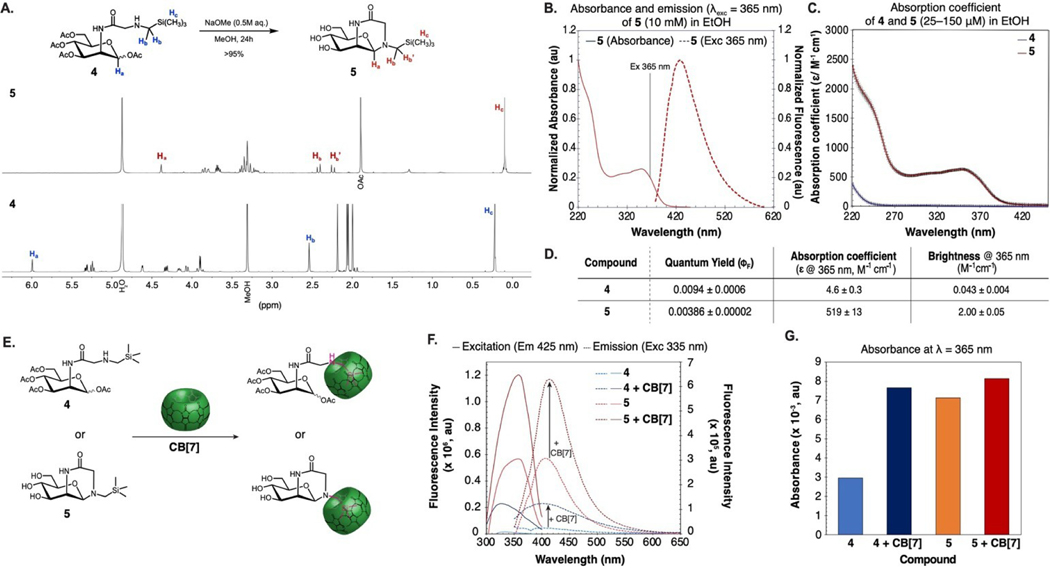
Figure 5.
Brightness of 4 is enhanced by cyclization and/or encapsulation with CB[7]. (A) 1H NMR of 4 before and after deacetylation. No purification was performed before the 1H NMR of 5 was obtained. (B) Absorption and emission spectra of 5 (10 mM) in EtOH. (C) Absorption coefficient of 4 and 5 at λ=220–450 nm. (D) Table of photophysical properties of 4 and 5. (E) Schematic depicting the complexation of CB[7] with 4 and 5 to increase fluorescence intensity. (F) Comparison of emissive properties: excitation spectra with emission at 425 nm and emission spectra with excitation at 335 nm of 4 (500 μM) and 5 (500 μM) in the presence or absence of CB[7] (500 μM) in H2O. (G) Absorbance at λ=365 nm of 4 (500 μM) and 5 (500 μM) in the presence or absence of CB[7] (500 μM) in H2O.