Abstract
Essential tremor (ET) is a disabling condition resulting from a dysfunction of cerebello-thalamo-cortical circuitry. Deep brain stimulation (DBS) or lesion of the ventral-intermediate thalamic nucleus (VIM) is an effective treatment for severe ET. Transcranial cerebellar brain stimulation has recently emerged as a non-invasive potential therapeutic option. Here, we aim to investigate the effects of high-frequency non-invasive cerebellar transcranial alternating current stimulation (tACS) in severe ET patients already operated for VIM-DBS. Eleven ET patients with VIM-DBS, and 10 ET patients without VIM-DBS and matched for tremor severity, were included in this double-blind proof-of-concept controlled study. All patients received unilateral cerebellar sham-tACS and active-tACS for 10 min. Tremor severity was blindly assessed at baseline, without VIM-DBS, during sham-tACS, during and at 0, 20, 40 min after active-tACS, using kinetic recordings during holding posture and action (‘nose-to-target’) task and videorecorded Fahn-Tolosa-Marin (FTM) clinical scales. In the VIM-DBS group, active-tACS significantly improved both postural and action tremor amplitude and clinical (FTM scales) severity, relative to baseline, whereas sham-tACS did not, with a predominant effect for the ipsilateral arm. Tremor amplitude and clinical severity were also not significantly different between ON VIM-DBS and active-tACS conditions. In the non-VIM-DBS group, we also observed significant improvements in ipsilateral action tremor amplitude, and clinical severity after cerebellar active-tACS, with a trend for improved postural tremor amplitude. In non-VIM-DBS group, sham- active-tACS also decreased clinical scores. These data support the safety and potential efficacy of high-frequency cerebellar-tACS to reduce ET amplitude and severity.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13311-023-01372-6.
Keywords: Essential tremor, Transcranial alternating current stimulation, Cerebellum, Deep brain stimulation, Motion capture
Introduction
Essential tremor (ET) is a frequent and disabling disorder with progressive worsening postural tremor of the arms, which seriously affects quality of life [1]. ET has been associated with pathological changes in the cerebellum [2], reduced function of the cerebellar cortex and abnormal oscillations within the cerebello-thalamo-cortical (CTC) network [2-10]. High-frequency deep brain stimulation (DBS) of the ventral intermediate nucleus of the thalamus (VIM), which receives afferent inputs from the cerebellar deep nuclei, is very effective in improving ET [11-13]. It can decrease tremor amplitude by up to 40–80%, by disrupting the abnormal rhythmic activity within the CTC network with low- and high-frequency thalamic oscillation reduction [14-16]. Although effective and well-tolerated [16], this technique is an invasive neurosurgical procedure, and decreasing responsiveness and the occurrence of side-effects could impact its benefits in the long-term [17, 18]. Recently, VIM lesioning using focused ultrasound has been reported to drastically improve ET symptoms with good tolerance [19, 20]. However, both treatments are of high cost with potential adverse events.
Non-invasive brain stimulation (NIBS) techniques targeting the cerebellum, such as repetitive transcranial magnetic stimulation (rTMS), theta burst stimulation (TBS) or trans-cutaneous direct current stimulation (tDCS), have also been tested in a few studies to manage ET [21-26]. Open-label studies and a recent meta-analysis suggested positive therapeutic effects of cerebellar NIBS for ET [21, 23], although double-blind proof-of-concept studies mainly failed to demonstrate its therapeutic interest of NIBS [24, 27, 28]. In two recent controlled trials, low-frequency rTMS applied over the cerebellum induced a significant improvement in tremor severity immediately after [25, 29]. Recently, transcranial alternating current stimulation (tACS) has emerged as a NIBS technique to manipulate intrinsic neuronal oscillations with externally applied rhythmic electrical frequencies, with after-effects that can persist over 1 h after current offset [30]. When applied over the cerebellum at 5 Hz or 300 Hz in healthy subjects, it led to an increase in cerebellar-brain inhibition (CBI), with no changes in the short-intracortical inhibition and short-afferent inhibition [31, 32]. Applied at 50 Hz it induced no changes [31] or a CBI weakening with better sequential tapping adaptation [32]. Lastly, phase-locked cerebellar tACS has also been shown to decrease tremor amplitude in a small sample of patients with ET [33] or with non-jerky dystonic tremor [34]. Finally, the fact that: 1) all these previous data highlight the potential benefits of cerebellar tACS in modulating CTC network functioning; 2) ET patients exhibited various abnormal neuronal oscillations within the CTC network, including very low and high frequency oscillations, which may reflect altered Purkinje cells GABAergic neurotransmission [35, 36, 37]; 3) high-frequency thalamic DBS dramatically improves ET severity in correlation with increased activation within the whole CTC network, intra-regional inhibition with decreased neuronal oscillations [17], strongly support the potential value of cerebellar tACS for ET management.
Here, we hypothesise that high-frequency tACS could improve tremor severity similarly to high-frequency VIM-DBS, by increasing GABAergic inputs to deep cerebellar nuclei, and subsequently modulate the CTC network excitability and neuronal oscillations. To test this hypothesis, we first studied the effects of high-frequency VIM-DBS and cerebellar tACS applied at the same frequency in ET patients previously treated by VIM-DBS. We also tested the effects of high-frequency cerebellar tACS in a group of ET patients not previously operated for VIM-DBS and matched for tremor severity.
Methods
Study Design and Patients
In this double-blind trial, we recruited patients from the Salpêtrière Hospital, Paris, France. All patients were assessed at the Paris Brain Institute. Two groups of patients were included. All patients were eligible for inclusion if they fulfilled ET diagnosis criteria according to the Task Force on tremor of the International Parkinson and Movement Disorders Society [38], were aged 18 to 75 years, had no other causes of tremor or neurological disorders, were affiliated to the French national health insurance or similar scheme, agreed to participate and provided written informed consent. An additional inclusion criterion was unilateral VIM-DBS for more than 6 months prior to this study for patients in the VIM-DBS group. This study received approval from the local ethics committee of Paris VI University and the French Ministry of Health. All patients gave written informed consent. The study was performed according to the Declaration of Helsinki and Good clinical practice guidelines, and was sponsored by the INSERM (C14-44). Clinical trial.gov registration: NCT02346409.
Procedures
Patients with VIM-DBS were first assessed with unilateral VIM-DBS ON with their usual stimulation parameters, and the DBS was then switched OFF for 3 h to avoid rebound effects due to sudden neurostimulation cessation. Then, all patients were assessed without treatment, OFF VIM-DBS for patients previously operated (baseline assessment). Then, each patient first received sham-tACS followed by active high-frequency tACS for 10 min each, without being informed of the order of application, and at 0, 20 and 40 min after current offset (Fig. 1). Anti-tremor drugs were stopped 7 days before the assessment in both groups, except for propranolol which was stopped the day before.
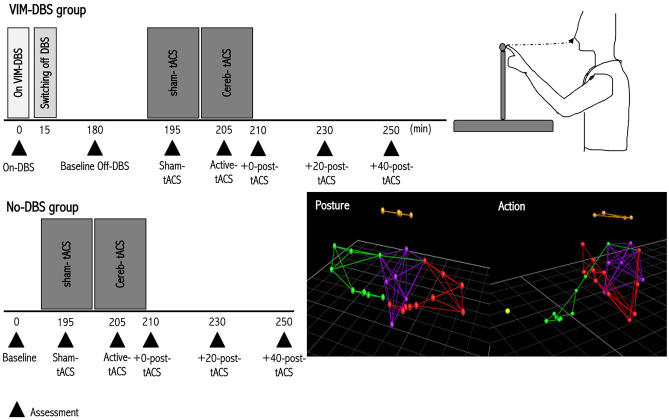
Fig. 1.
Study design. For VIM-DBS patients (upper panel, left part), assessments were performed first ON-VIM DBS, then after ceasing VIM-DBS for 3 h (OFF DBS/baseline). In the OFF-DBS condition, sham-tACS was applied for 10 min followed by active-cerebellar tACS for 10 min. Patients were assessed during sham-tACS, during active-tACS and at 0, 20 and 40 min following current offset. The same protocol was applied for the patients not previously operated for VIM-DBS, i.e. No-DBS Group (lower panel, left part). We measured tremor amplitude using kinematic arm recordings using the VICON® 3D motion capture system with 29 reflective markers positioned bilaterally on the arms, head and trunk during posture holding and action with the 'nose-to-target' task (right part, lower and upper panels, respectively)
We performed assessments of tremor severity by using the Fahn-Tolosa-Marin (FTM) scale [39], and kinetic recordings of arm tremor using a VICON® 3D motion capture system with 29 reflective markers positioned bilaterally on the arms, head and trunk (Fig. 1).
Cerebellar tACS was administrated to patients at rest, seated in a chair, and positioned in front of a table. tACS was delivered by a battery-driven stimulator (NeuroConn, DC-Stimulator Plus, CE) connected to a pair of saline-soaked square-shaped sponge electrodes (9 cm2). One electrode was positioned over the posterior cerebellum (3 cm lateral to the inion), contralateral to VIM-DBS, whereas the return electrode was positioned over the contralateral cheekbone. Electrodes were fixed by elastic bands. For the control group, we applied cerebellar tACS ipsilaterally to the most severe side with a similar set-up. During active stimulation, tACS was delivered at a frequency of 130 to 180 Hz matched to the chronic VIM-DBS stimulation frequency in the VIM-DBS group; and at 130 Hz in the No-DBS group. The stimulation intensity was set at 1 mA and was applied over a 10-min period resulting in a current density of 0.11 mA/cm2 [40] and a delivered total charge of 0.066 C/cm2. Sham-tACS was also applied for a 10 min period starting with delivery of a 1 mA current but for 10 s only. All patients reported a moderate local paraesthesia at the beginning of both sham- and active-tACS and they were unable to differentiate the two conditions.
Randomization and Masking
This proof-of-concept trial was double-blind and sham-controlled (Fig. 1). All the assessments were recorded and videorecorded, and rated in random order by two blinded investigators at the end of the study (ZK performed the clinical scale rating and OC the kinetics analysis), all sequences having been previously coded by a blinded clinical research assistant.
Outcomes
The predefined primary outcome was tremor amplitude, as assessed by the displacements of the markers positioned on the arms during a 5 s holding posture (arms folded, wrists slightly extended, fingers apart). Secondary endpoints included: i) the movement duration for performance of the ‘nose-to-target’ task with a marker placed on a support on the table at a fixed position with 5 round trips (Fig. 1), and the displacement of the finger marker within a 2cm2 area around the target (reflecting the pointing precision); ii) the clinical severity with the total score of the FTM scale, with part A rating the tremor severity of each body part in different posture and action tasks and part B rating the tremor severity during handwriting, drawing and pouring (we did not assess orthostatic tremor in part A, nor pouring in part B); and iii) the safety and tolerance of the procedure, as assessed by the occurrence of adverse events, serious adverse events or discomfort.
Statistical Analysis
In this study, we aimed to examine the changes in tremor severity with active cerebellar high frequency tACS. In line with previous data obtained after cerebellar NIBS [21, 23, 25], we expected a decrease of 25% (SD 15%) in the tremor amplitude during active tACS with respect to baseline. With this assumption, our 10 patients should enable us to have a power of 90% with an alpha error rate of 5% (Wilcoxon signed-rank test).
The main outcome criterion was the sum of the displacements of the arm markers during posture holding (postural tremor) at the different timepoints. Secondary outcomes were the time required to perform the ‘nose-to-target’ task (action tremor) and the precision of targeting (measured by the displacements of the finger markers in the 2 cm2 area around the target, Fig. 1), and the FTM scores. For this purpose, we modelled each outcome using a linear mixed model. We included the treatment condition (baseline or OFF VIM-DBS, ON VIM-DBS, during sham-tACS, during and 0, 20, 40 min after active-tACS) and patient intercepts as random effects. We used R (R, version 3.3.1, R Core Development Team, FactoMineR package) for statistical analyses, with the LmerTest package for the linear mixed-effect model computation. We used a significance threshold of 0.05 with Bonferroni correction and p-values were adjusted using the false-discovery-rate method.
Results
Patient Characteristics
Between February 2015 and July 2018, we included 11 patients with ET previously operated for VIM-DBS (3F/8 M) and 10 patients with ET not previously operated for VIM-DBS (2F/8 M) that were not significantly different for age (p = 0.24), disease duration (p = 0.48) or tremor severity (p = 0.95, Tables 1).
Table 1.
Baseline demographic and clinical characteristics of patients with Essential tremor
| Patient | Sex, age (yrs) | Disease duration (yrs) | Time since surgery for VIM-DBS (months) | FTM total score (baseline)a | Medication (dosage/day) |
tACS frequency (Hz) |
VIM-DBS settings (contact/pulse width/amplitude) |
|
|---|---|---|---|---|---|---|---|---|
| ET with VIM-DBS | 01 | M, 61 | 10 | 48 | 41 | – | 180 | 1–2-C + / 60 μs/3.8 V |
| 02 | M, 73 | 18 | 26 | 35 | Propanolol 100 mg | 130 | 1–2 + /120 μs/3.5 V | |
| 03 | M, 67 | 12 | 24 | 26 | Topiramate 50 mg, Primidone 250 mg | 130 | 1-C + /60 μs/3.7 V | |
| 04 | F, 47 | 27 | 15 | 11 | Propanolol 80 mg, Gabapentin 300 mg | 130 | 2-C + /60 μs/3.0 V | |
| 05 | M, 56 | 40 | 12 | 19 | – | 180 | 0–1 + / 90 μs/4 V | |
| 06 | M, 29 | 12 | 10 | 17 | – | 130 | 0-C + /60 μs us/3.0 V | |
| 07 | M, 69 | 49 | 30 | 36 | – | 130 | 2-C + /60 μs/2.8 V | |
| 08 | M, 69 | 22 | 93 | 18 | – | 180 | 1–0 + /60 μs/4.0 V | |
| 09 | F, 41 | 26 | 12 | 28 | Propanolol 40 mg, Topiramate 25 mg, Gabapentin 300 mg | 130 | 0-C + /60 μs/2.8 V | |
| 10 | F, 72 | 16 | 55 | 26 | – | 130 | 1–2 + /60 μs/3.6 V | |
| 11 | M, 44 | 34 | 36 | 42 | Topiramate 50 mg | 180 | 1-C + /60 μs/3.9 V | |
| Mean (SD) | 57.1 (14.8) | 24.2 (12.6) | 32.8 (24.9) | 27.2 (10.3) | ||||
| ET without VIM-DBS | 12 | M, 68 | 46 | – | 34 | Propanolol 120 mg, Gabapentin 600 mg | 130 | |
| 13 | M, 70 | 12 | – | 26 | Propanolol 100 mg | 130 | ||
| 14 | M, 74 | 46 | – | 23 | Topiramate 50 mg, Primidone 50 mg | 130 | ||
| 15 | M, 66 | 52 | – | 28 | – | 130 | ||
| 16 | M, 52 | 12 | – | 21 | Propanolol 60 mg | 130 | ||
| 17 | M, 71 | 17 | – | 19 | Propanolol 40 mg | 130 | ||
| 18 | M, 71 | 42 | – | 29 | Propanolol 40 mg | 130 | ||
| 19 | F, 71 | 15 | – | 23 | Propanolol 100 mg | 130 | ||
| 20 | M, 61 | 53 | – | 32 | Propanolol 80 mg | 130 | ||
| 21 | F, 42 | 12 | – | 33 | – | 130 | ||
| Mean (SD) | 64.6 (10.2) | 30.7 (18.3) | – | 26.8 (5.2) |
VIM-DBS: Ventral Intermediate Thalamic Deep Brain Stimulation, F: female, M: male
aOFF VIM-DBS for at least 3 h for patients with VIM DBS
Effects of Cerebellar tACS on ET Severity in the VIM-DBS Group
Postural Tremor Amplitude
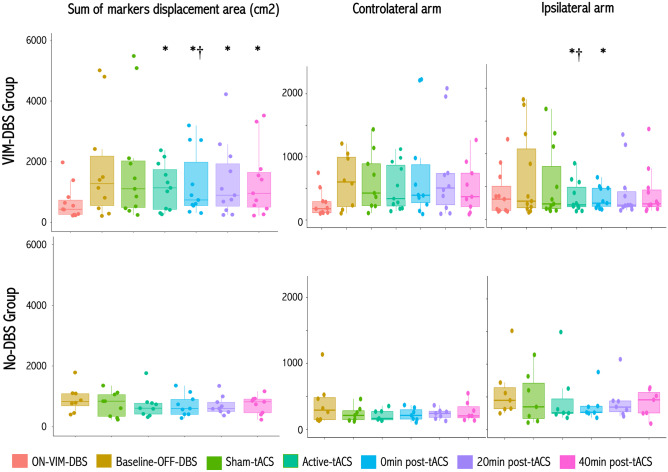
In the active-tACS vs OFF-VIM-DBS condition, the postural tremor amplitude decreased significantly during and at 0, 20 and 40 min following stimulation (p = 0.02, p = 0.03, p = 0.04 and p = 0.04, respectively, Fig. 2, see supplementary video). There was no significant difference in postural tremor amplitude during active-tACS vs ON VIM-DBS condition (p = 0.26, Fig. 2), nor during sham-tACS vs OFF VIM-DBS (p = 0.26). Comparing the effects of active-tACS between arms, we found that the effects of active-tACS were only significant for the ipsilateral arm during and at 0 min following active-tACS relative to OFF VIM-DBS (p = 0.02 and p = 0.02, respectively), and relative to sham-tACS (p = 0.03), with no significant difference for the contralateral arm (Fig. 2, see supplementary video).
Fig. 2.
Effects of active- and sham-tACS at high-frequency on postural tremor. Box plots for postural tremor amplitude as measured by the sum of the displacements of the two arm markers (left panels) during posture holding in ET patients with VIM-DBS (upper panels), and No-DBS patient group (lower panels). Postural tremor amplitude for the contralateral (middle panels) and ipsilateral (right panels) arms. *P < 0.05 relative to baseline condition; †P < 0.05 relative to sham-tACS
Action Tremor Amplitude
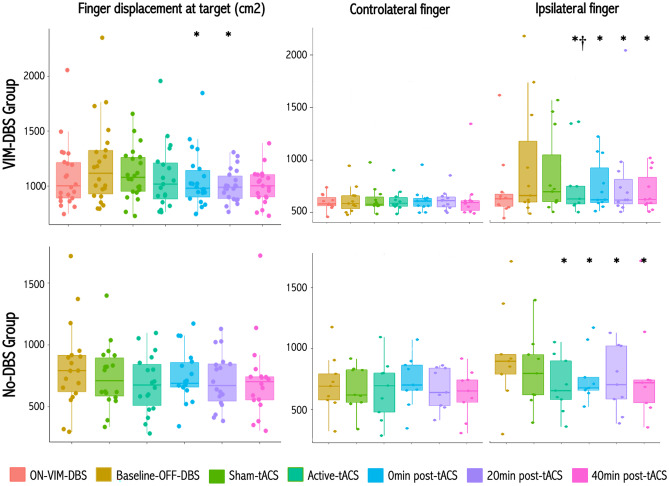
In the active-tACS vs OFF-VIM-DBS condition, finger displacement within the 2cm2 area around the target during the ‘nose-to-target’ task was significantly lower at 0 and 20 min following stimulation (p = 0.04 and p = 0.008, respectively), with a trend during and at 40 min following stimulation (p = 0.05). These effects were only significant for the ipsilateral arm during, at 0, 20 and 40 min following stimulation (p = 0.002, p = 0.045, p = 0.005 and p = 0.008, respectively, Fig. 2). Finger displacement of the ipsilateral arm was also significantly lower with active-tACS vs sham-tACS (p = 0.02, Fig. 2). Finger displacement was not significantly different between active tACS vs the ON VIM-DBS condition (p = 0.32), and between sham-tACS vs the OFF VIM-DBS condition (p = 0.26, Fig. 3).
Fig. 3.
Effects of active- and sham-tACS at high-frequency on action tremor. Box plots for action tremor amplitude as measured by the sum of the displacements of the finger in the 2cm2 area around the target, for the two arms (left graphs), the contralateral (middle panels) and ipsilateral (right panels) arms, during the 'nose-to-target' task in ET patients with VIM-DBS (upper panel), and No-DBS patient group (lower panel ). *P < 0.05 relative to baseline condition; †P < 0.05 relative to sham-tACS
The task duration was also significantly lower in the active-tACS vs OFF-VIM-DBS condition (p = 0.012, p < 10–4, p < 10–4, p < 10–4, respectively for during-stimulation and at 0, 20 and 40 min after, not shown), but only in the ipsilateral arm (p = 0.003, p < 10–4, p < 10–4, p < 10–4 ipsilaterally; contralaterally the respective values were p = 0.88, p = 0.05, p = 0.20, p = 0.20).
Clinical ET Severity
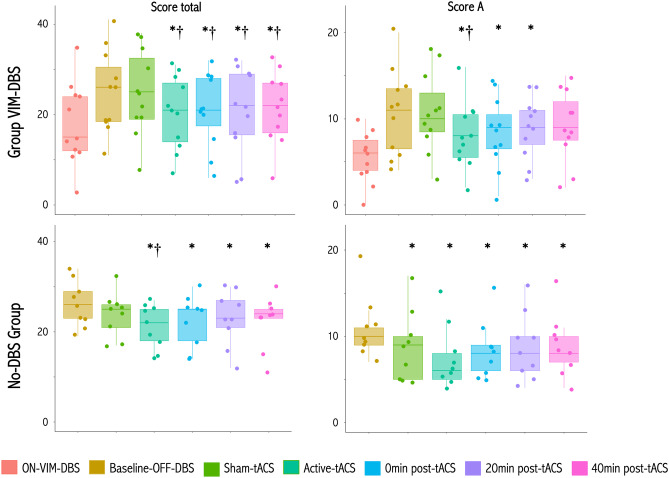
The FTM total score (blinded video scoring) was significantly lower during active-tACS and after 0, 20, 40 min vs both OFF VIM-DBS (p = 0.006, p = 0.010, p = 0.007, p = 0.02, respectively, Fig. 4) and sham tACS (p = 0.006, p = 0.010, p = 0.007, p = 0.02, respectively, Fig. 4) conditions (Table 2). There was no significant difference in the FTM total score during active-tACS vs the ON VIM-DBS condition (p = 0.10, Fig. 4), nor during sham-tACS vs the OFF VIM-DBS condition (p = 1.0, Fig. 4).
Fig. 4.
Effects of active- and sham-tACS at high frequency on clinical tremor severity. Box plots for the severity of tremor as measured by the FTM total and by the FTM subscore A in ET patients with VIM-DBS (upper panels) and No-DBS patient group (lower panels). *P < 0.05 relative to baseline condition; †P < 0.05 relative to sham-tACS
Table 2.
Effects of high-frequency VIM-DBS, active-tACS and sham-tACS of the cerebellum on clinical tremor severity in patients with Essential Tremor
| ON VIM-DBSa | Baseline (OFF VIM-DBSa) | Sham-tACS | Active-tACS | Change between baseline and Sham-tACS | Change between baseline and Active-tACS | Change between Active and Sham-tACS | |
|---|---|---|---|---|---|---|---|
| FTM score A | |||||||
| VIM-DBS group | 5.6 (3.0)* | 11.5 (.8) | 11.3 (4.3) | 8.1 (3.8) | −0.2 (3.0) | −3.4 (3.4)* | −3.2 (2.2)** |
| No-DBS group | – | 11.2 (3.6) | 9.5 (4.3) | 8.1 (3.8) | −1.7 (2.8)* | −3.1 (2.1)* | −1.4 (1.6) |
| FTM score B | |||||||
| VIM-DBS group | 11.6 (5.2) | 15.6 (6.4) | 14.5 (8.1) | 13.6 (6.9) | −1.0 (3.3) | −1.9 (2.5) | −0.9 (3.7) |
| No-DBS group | – | 15.6 (3.4) | 15.3 (3.3) | 14.0 (3.9) | −0.3 (1.8) | −1.6 (2.1) | −1.3 (2.3) |
| FTM total score | |||||||
| VIM-DBS group | 17.2 (7.7)* | 27.2 (10.3) | 26.7 (10.1) | 21.5 (9.0) | −0.5 (1.2) | −5.6 (5.1)* | −5.2 (3.6)** |
| No-DBS group | – | 26.8 (5.3) | 24.8 (5.9) | 22.1 (5.3) | −2.0 (2.9) | −4.7 (3.2)* | −2.7 (3.2)** |
Values are mean (SD); FTM = Fahn-Tolosa-Marin score
afor patients of the VIM-DBS group only
*p < 0.05 relative to Baseline (OFF VIM-DBS) condition; **p < 0.05 between Sham- and Active-tACS condition (linear mixed model)
The subscore A (tremor severity) was significantly lower during active tACS and after 0, 20 min vs OFF-VIM-DBS (p = 0.003, p = 0.005, p = 0.045, respectively), and during active-tACS vs sham-tACS (p = 0.003, Fig. 4 and Table 2) conditions. The subscore A did not change significantly during sham-tACS vs the baseline (p = 0.90, Fig. 4). We found no significant changes in the subscore B (writing, drawing) with sham or active-tACS vs OFF VIM-DBS (all p values > 0.35, Table 2).
Effects of Cerebellar tACS on ET Severity in the No-DBS Group
Postural Tremor Amplitude
The postural tremor amplitude did not change significantly during and after active-tACS relative to both baseline (p = 0.15) and sham-tACS conditions (p = 0.15), and in the two arms (Fig. 2).
Action Tremor Amplitude
In the active-tACS vs baseline condition, finger displacement within the 2 cm2 area around the target assessed during the ‘nose-to-target’ task was significantly lower during and 0, 20 and 40 min after stimulation, but on the ipsilateral side only (p < 10–4, p = 0.02, p = 0.01, p = 0.04, for during, and 0, 20 and 40 min after active tACS, respectively, Fig. 3), with a trend in comparison to the sham-tACS (p = 0.10). We found no significant change in finger displacements with sham-tACS vs baseline condition, regardless of the arm considered (Fig. 3, p = 0.13 and p = 0.71 for ipsilateral and contralateral arms, respectively, Fig. 3).
The ‘nose-to-target’ task duration was also significantly lower during active-tACS and at 0, 20, 40 min following stimulation vs baseline (p < 10–4, p < 10–4, p < 10–4, and p < 10–4, respectively). This effect was significant for both sides (not shown), and relative to sham-tACS for the ipsilateral arm (p = 0.04, and p = 0.11 for the contralateral arm, not shown).
Clinical ET Severity
The FTM total score was significantly lower during active tACS and at 0, 20, 40 min following stimulation vs baseline (p < 10–4, p = 0.03, p = 0.018 and p = 0.003, respectively, Fig. 4), and during active-tACS vs sham-tACS (p = 0.045, Fig. 4) conditions (Table 2). During sham-tACS vs baseline, there was no significant difference in the FTM total score (p = 0.06, Fig. 4). With respect to baseline, the subscore A was significantly lower during and at 0, 20, 40 min following active-tACS (p < 10–4, p = 0.01, p = 0.02 and p = 0.02, respectively, Fig. 4) and also with sham-tACS (p = 0.02, Fig. 4 and Table 2). We found no significant changes for the subcore B with either sham or active-tACS vs baseline (all p values > 0.35, Table 2).
Safety and Tolerance of the Procedure
No adverse or serious adverse event occurred during the trial. Both sham and active-tACS were generally well-tolerated regardless of the group. Two patients with VIM-DBS described mild transient paraesthesia of the ipsilateral arm occurring at current onset (during both the sham and active tACS conditions), and one patient in the control group described moderate transient paraesthesia under the skull electrode during both sham- and active tACS conditions.
Discussion
In this proof-of-concept double-blind sham-controlled study, we assessed for the first time the effects of high-frequency cerebellar tACS on ET patients treated with VIM-DBS; and in ET patients with similar tremor severity with no VIM-DBS, taken as controls. Using objective measurements (kinetic measures using VICON® 3D motion capture) and blind assessments of the FTM scores by independent examiners, we found a significant improvement in kinetic and clinical measures with active-cerebellar tACS. The effects were comparable to those of VIM-DBS for managing posture tremor amplitude and reducing clinical severity, and only present in the ipsilateral arm. In the control group of ET patients not previously treated with VIM-DBS, we also found a significant improvement in the ‘nose-to-target’ task parameters in the ipsilateral arm, and in tremor clinical severity after active-cerebellar tACS.
Interestingly, we observed variable effects across patients of both groups, with in addition, an absence of decreased tremor severity (total FTM score) with active-tACS in 4 out of our 21 patients (19%), and no change in writing tremor for about one third of patients (score B). Such interindividual variability in cerebellar NIBS has been reported in previous NIBS trials [26], also with individual phase-locked tACS [33]. These variable effects of active-tACS could be due to differences in clinical characteristics, such as disease duration or age [26], features of the tremor movement [33], electrode–skin capacitance, or individual cerebellar-cortex anatomical and functional connectivity at baseline [9, 10].
The physiological effects of non-invasive brain stimulation of the cerebellar cortex are not fully understood. Here, we hypothesised that high frequency tACS would modify cerebellar cortex excitability leading to subsequent changes in the pathological oscillatory activity within the CTC network. The fact that we found similar effects between VIM-DBS and active cerebellar tACS, applied at the same stimulation frequency, would favour this hypothesis. In ET patients, a possible progressive neurodegeneration of Purkinje cells has been reported with reduced activity of these cells [2, 41], leading to a deficit in GABAergic inputs to the deep cerebellar nuclei neurons, and pathological oscillatory activities within the CTC network activity [7]. Previous studies demonstrated an increased theta-alpha band activity and high-frequency oscillations in the VIM of ET patients [37, 42, 43], with, in addition, an abnormal thalamocortical low-frequency oscillation phase-amplitude coupling [44, 45], a mechanism mediating movement execution [46]. With high-frequency VIM-DBS, tremor suppression was found to be correlated to decreased thalamic neuronal firing and low-frequency oscillations [47-49], and modulation of both olivocerebellar and thalamocortical circuits with activation of the contralateral cerebellar cortex, deep cerebellar nuclei and motor cortex [17, 50]. These data suggests that VIM-DBS could lead to a partial reestablishment of CTC functioning, and act as a filter to uncouple thalamo-cortical from cortico-spinal reflex loops [48]. Applied at the cerebellar cortex, 5 Hz tACS has been shown to increase cerebello-cortex inhibition in healthy subjects [31], and low-frequency phase-locked cerebellar tACS has also been reported to decrease tremor amplitude in ET patients [33]. Conversely, cathodal cerebellar tDCS, thought to inhibit cerebellar cortical activity, does not significantly reduce tremor [24]. Lastly, when applied over the cerebellum, 300 Hz tACS has been shown to increase cerebellum-brain inhibition [32], and 140 Hz tACS applied over M1 to increase cortical excitability [30]. Finally, all these previous studies and our results suggest that high frequency tACS applied over the cerebellum may increase Purkinje cell excitability with, in consequence, a modulation of the CTC network oscillatory activity and tremor reduction.
This study has some limitations. First, we cannot fully exclude the possibility that our findings on VIM-DBS ET patients resulted from an artifact due to the setup used with the cerebellar tACS, since current may have flowed through the DBS electrode. However, the facts that kinetic and functional improvements persisted for up to 40 min after current offset, were also found in non-operated ET patients, and more pronounced for the arm ipsilateral to the stimulated cerebellum do not favour this hypothesis. Second, we had no direct evidence that allowed us to determine which part of the cerebellum could be affected by the tACS nor its physiological effects on cerebellar activity. In our protocol, we positioned stimulating electrodes as recommended in previous studies using DC stimulation [51, 52] and the effects observed across patients led us to suppose that the posterior cerebellum was actively modulated. Third, although a strength of the study was the sham-controlled design, sham-tACS was always applied prior to active-tACS which may have primed the effects of active-tACS, and we cannot totally rule out the possibility that patients felt some differences between sham-tACS and active-tACS without mentioning it. We chose this experimental design for the comfort of the patients as the anti-tremor drugs were stopped 7 days preceding the assessment for all patients and stimulation was turned OFF for several hours for VIM-DBS patients, with a return to the pre-operative tremor disability. In addition, our primary outcome was the comparison of active-tACS vs VIM-DBS, and not per se sham vs active-tACS. We also observed some significant changes in kinetics parameters in our control ET patients group with sham-tACS suggesting a possible placebo effect in these patients, as has been previously reported in ET patients using NIBS [28, 29]. However, the fact that we found significant differences in blinded clinical scores between active and sham-tACS with better effects for the ipsilateral arm, and no effect of sham tACS in the VIM-DBS group, may indicate that active tACS is indeed more efficient than sham-tACS to decrease tremor severity [29].
Conclusions
Overall, this proof-of-concept study supports the fact that high-frequency cerebellar tACS could modulate the CTC network, with objective changes in the postural and kinetic characteristics of essential tremor. In addition, it demonstrates the safety and potential benefits of high-frequency cerebellar tACS for ET patients. Further studies are needed to assess the value of repeated sessions for long-lasting effects, determine the optimal stimulation settings. This needs to be done in relation to individual tremor characteristics [33] and anatomical and functional cerebellar-cortex connectivity [9, 23], before we can propose this technique for patients with tremor insufficiently controlled by medical treatment.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We extend our deepest thanks to the patients who participated in this study. We also thank the nurses and administrative staff of the Clinical Investigation Centre and S Mehdi for their help in the conduct of the study.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Funding
This study was supported by a grant from the International Essential tremor foundation and ‘Investissements d’avenir’ (ANR-10-IAIHU-06). This study was sponsored by INSERM (C14-44).
Data Availability
All relevant data are within the article. Requests for anonymized data should be sent to M.L. Welter at the Paris Brain Institute, 75013 Paris, France.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Claire Olivier and Jean-Charles Lamy contributed equally to this paper.
References
- 1.Louis ED, Ottman R, Hauser WA. How common is the most common adult movement disorder? estimates of the prevalence of essential tremor throughout the world. Mov Disord. 1998;13:5–10. doi: 10.1002/mds.870130105. [DOI] [PubMed] [Google Scholar]
- 2.Louis ED, Faust PL, Vonsattel JP, et al. Neuropathological changes in essential tremor: 33 cases compared with 21 controls. Brain. 2007;130:3297–3307. doi: 10.1093/brain/awm266. [DOI] [PubMed] [Google Scholar]
- 3.Deuschl G, Elble RJ. The pathophysiology of essential tremor. Neurology. 2000;54:S14–20. doi: 10.1212/WNL.54.4.14A. [DOI] [PubMed] [Google Scholar]
- 4.Placantonakis DG, Bukovsky AA, Zeng XH, et al. Fundamental role of inferior olive connexin 36 in muscle coherence during tremor. Proc Natl Acad Sci U S A. 2004;101:7164–7169. doi: 10.1073/pnas.04003221010400322101[pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pedrosa DJ, Reck C, Florin E, et al. Essential tremor and tremor in Parkinson’s disease are associated with distinct “tremor clusters” in the ventral thalamus. Exp Neurol. 2012;237:435–443. doi: 10.1016/j.expneurol.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 6.Gallea C, Popa T, García-Lorenzo D, et al. Intrinsic signature of essential tremor in the cerebello-frontal network. Brain. 2015;138:2920–2933. doi: 10.1093/brain/awv171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Filip P, Lungu OV, Manto M-U, et al. Linking essential tremor to the cerebellum: physiological evidence. Cerebellum. 2016;15:774–780. doi: 10.1007/s12311-015-0740-2. [DOI] [PubMed] [Google Scholar]
- 8.Fang W, Chen H, Wang H, et al. Essential tremor is associated with disruption of functional connectivity in the ventral intermediate Nucleus-Motor Cortex–Cerebellum circuit. Hum Brain Mapp. 2016;37:165–178. doi: 10.1002/hbm.23024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hanajima R, Tsutsumi R, Shirota Y, et al. Cerebellar dysfunction in essential tremor. Mov Disord. 2016;31:1230–1234. doi: 10.1002/mds.26629. [DOI] [PubMed] [Google Scholar]
- 10.Pinto AD, Lang AE, Chen R. The cerebellothalamocortical pathway in essential tremor. Neurology. 2003;60:1985–1987. doi: 10.1212/01.wnl.0000065890.75790.29. [DOI] [PubMed] [Google Scholar]
- 11.Deuschl G, Raethjen J, Hellriegel H, et al. Treatment of patients with essential tremor. Lancet Neurol. 2011;10:148–161. doi: 10.1016/S1474-4422(10)70322-7. [DOI] [PubMed] [Google Scholar]
- 12.Kumar R, Lozano AM, Sime E, et al. Long-term follow-up of thalamic deep brain stimulation for essential and parkinsonian tremor. Neurology. 2003;61:1601–1604. doi: 10.1212/01.WNL.0000096012.07360.1C. [DOI] [PubMed] [Google Scholar]
- 13.Bardinet E, Belaid H, Grabli D, et al. Thalamic stimulation for tremor: can target determination be improved? Mov Disord. 2011;26:307–312. doi: 10.1002/mds.23448. [DOI] [PubMed] [Google Scholar]
- 14.Limousin P, Speelman JD, Gielen F, et al. Multicentre European study of thalamic stimulation in parkinsonian and essential tremor. J Neurol Neurosurg Psychiatry. 1999;66:289–296. doi: 10.1136/jnnp.66.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schuurman PR, Bosch DA, Bossuyt PM, et al. A comparison of continuous thalamic stimulation and thalamotomy for suppression of severe tremor. N Engl J Med. 2000;342:461–468. doi: 10.1056/NEJM200002173420703. [DOI] [PubMed] [Google Scholar]
- 16.Rehncrona S, Johnels B, Widner H, et al. Long-term efficacy of thalamic deep brain stimulation for tremor: double-blind assessments. Mov Disord. 2003;18:163–170. doi: 10.1002/mds.10309. [DOI] [PubMed] [Google Scholar]
- 17.Gibson WS, Jo HJ, Testini P, et al. Functional correlates of the therapeutic and adverse effects evoked by thalamic stimulation for essential tremor. Brain. 2016;139:2198–2210. doi: 10.1093/brain/aww145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fasano A, Herzog J, Raethjen J, et al. Gait ataxia in essential tremor is differentially modulated by thalamic stimulation. Brain. 2010;133:3635–3648. doi: 10.1093/brain/awq267. [DOI] [PubMed] [Google Scholar]
- 19.Iorio-Morin C, Yamamoto K, Sarica C, et al. Bilateral focused ultrasound thalamotomy for essential tremor (BEST-FUS Phase 2 Trial) Mov Disord. 2021;36:2653–2662. doi: 10.1002/mds.28716. [DOI] [PubMed] [Google Scholar]
- 20.Abe K, Horisawa S, Yamaguchi T, et al. Focused ultrasound thalamotomy for refractory essential tremor: A Japanese multicenter single-arm study. Neurosurgery. 2021;88:751–757. doi: 10.1093/neuros/nyaa536. [DOI] [PubMed] [Google Scholar]
- 21.Kang N, Cauraugh JH. Does non-invasive brain stimulation reduce essential tremor? A systematic review and meta-analysis. PLoS One. 2017;12:e0185462. doi: 10.1371/journal.pone.0185462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Latorre A, Rocchi L, Berardelli A, et al. The use of transcranial magnetic stimulation as a treatment for movement disorders: A critical review. Mov Disord. 2019;34:769–782. doi: 10.1002/mds.27705. [DOI] [PubMed] [Google Scholar]
- 23.Popa T, Russo M, Vidailhet M, et al. Cerebellar rTMS stimulation may induce prolonged clinical benefits in essential tremor, and subjacent changes in functional connectivity: an open label trial. Brain Stimul. 2013;6:175–179. doi: 10.1016/j.brs.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 24.Gironell A, Martinez-Horta S, Aguilar S, et al. Transcranial direct current stimulation of the cerebellum in essential tremor: a controlled study. Brain Stimul. 2014;7:491–492. doi: 10.1016/j.brs.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 25.Gironell A, Kulisevsky J, Lorenzo J, et al. Transcranial magnetic stimulation of the cerebellum in essential tremor: a controlled study. Arch Neurol. 2002;59:413–417. doi: 10.1001/archneur.59.3.413. [DOI] [PubMed] [Google Scholar]
- 26.Maas RPPWM, Helmich RCG, van de Warrenburg BPC. The role of the cerebellum in degenerative ataxias and essential tremor: Insights from noninvasive modulation of cerebellar activity. Mov Disord. 2020;35:215–27. doi: 10.1002/mds.27919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bologna M, Rocchi L, Leodori G, et al. Cerebellar continuous theta burst stimulation in essential tremor. Cerebellum. 2015;14:133–141. doi: 10.1007/s12311-014-0621-0. [DOI] [PubMed] [Google Scholar]
- 28.Olfati N, Shoeibi A, Abdollahian E, et al. Cerebellar repetitive transcranial magnetic stimulation (rTMS) for essential tremor: A double-blind, sham-controlled, crossover, add-on clinical trial. Brain Stimul. 2020;13:190–196. doi: 10.1016/j.brs.2019.10.003. [DOI] [PubMed] [Google Scholar]
- 29.Shin H-W, Hallett M, Sohn YH. Cerebellar repetitive transcranial magnetic stimulation for patients with essential tremor. Parkinsonism Relat Disord. 2019;64:304–307. doi: 10.1016/j.parkreldis.2019.03.019. [DOI] [PubMed] [Google Scholar]
- 30.Moliadze V, Antal A, Paulus W. Boosting brain excitability by transcranial high frequency stimulation in the ripple range. J Physiol. 2010;588:4891–4904. doi: 10.1113/jphysiol.2010.196998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spampinato D, Avci E, Rothwell J, et al. Frequency-dependent modulation of cerebellar excitability during the application of non-invasive alternating current stimulation. Brain Stimul. 2021;14:277–283. doi: 10.1016/j.brs.2021.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Naro A, Leo A, Russo M, et al. Does transcranial alternating current stimulation induce cerebellum plasticity? Feasibility, safety and efficacy of a novel electrophysiological approach. Brain Stimul. 2016;9:388–395. doi: 10.1016/j.brs.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 33.Schreglmann SR, Wang D, Peach RL, et al. Non-invasive suppression of essential tremor via phase-locked disruption of its temporal coherence. Nat Commun. 2021;12:363. doi: 10.1038/s41467-020-20581-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nieuwhof F, Toni I, Buijink AWG, et al. Phase-locked transcranial electrical brain stimulation for tremor suppression in dystonic tremor syndromes. Clin Neurophysiol. 2022;140:239–250. doi: 10.1016/j.clinph.2022.03.020. [DOI] [PubMed] [Google Scholar]
- 35.De Zeeuw CI, Hoebeek FE, Schonewille M. Causes and consequences of oscillations in the cerebellar cortex. Neuron. 2008;58:655–658. doi: 10.1016/j.neuron.2008.05.019. [DOI] [PubMed] [Google Scholar]
- 36.de Solages C, Szapiro G, Brunel N, et al. High-frequency organization and synchrony of activity in the purkinje cell layer of the cerebellum. Neuron. 2008;58:775–788. doi: 10.1016/j.neuron.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 37.Schnitzler S, Hartmann CJ, Groiss SJ, et al. Occurrence of thalamic high frequency oscillations in patients with different tremor syndromes. Clin Neurophysiol. 2018;129:959–966. doi: 10.1016/j.clinph.2018.01.073. [DOI] [PubMed] [Google Scholar]
- 38.Bhatia KP, Bain P, Bajaj N, et al. Consensus Statement on the classification of tremors. from the task force on tremor of the International Parkinson and Movement Disorder Society. Mov Disord. 2018;33:75–87. doi: 10.1002/mds.27121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fahn S, Tolosa E, Marin C. Clinical rating scale for tremor. In: Jankovic J, Tolosa E, editors. Parkinson’s disease and movement disorders. 2. Baltimore: Williams and Wilkins; 1993. pp. 271–280. [Google Scholar]
- 40.Herzog R, Berger TM, Pauly MG, et al. Cerebellar transcranial current stimulation - An intraindividual comparison of different techniques. Front Neurosci. 2022;16:987472. doi: 10.3389/fnins.2022.987472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Louis ED. From neurons to neuron neighborhoods: the rewiring of the cerebellar cortex in essential tremor. Cerebellum. 2014;13:501–512. doi: 10.1007/s12311-013-0545-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brodkey JA, Tasker RR, Hamani C, et al. Tremor cells in the human thalamus: differences among neurological disorders. J Neurosurg. 2004;101:43–47. doi: 10.3171/jns.2004.101.1.0043. [DOI] [PubMed] [Google Scholar]
- 43.Kane A, Hutchison WD, Hodaie M, et al. Enhanced synchronization of thalamic theta band local field potentials in patients with essential tremor. Exp Neurol. 2009;217:171–176. doi: 10.1016/j.expneurol.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 44.Pedrosa DJ, Quatuor E-L, Reck C, et al. Thalamomuscular coherence in essential tremor: hen or egg in the emergence of tremor? J Neurosci. 2014;34:14475–14483. doi: 10.1523/JNEUROSCI.0087-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kondylis ED, Randazzo MJ, Alhourani A, et al. Movement-related dynamics of cortical oscillations in Parkinson’s disease and essential tremor. Brain. 2016;139:2211–2223. doi: 10.1093/brain/aww144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Opri E, Cernera S, Okun MS, et al. The functional role of thalamocortical coupling in the human motor network. J Neurosci. 2019;39:8124–8134. doi: 10.1523/JNEUROSCI.1153-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Opri E, Cernera S, Molina R, et al. Chronic embedded cortico-thalamic closed-loop deep brain stimulation for the treatment of essential tremor. Sci Transl Med. 2020;12:eaay7680. doi: 10.1126/scitranslmed.aay7680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Milosevic L, Kalia SK, Hodaie M, et al. Physiological mechanisms of thalamic ventral intermediate nucleus stimulation for tremor suppression. Brain. 2018;141:2142–2155. doi: 10.1093/brain/awy139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ferleger BI, Houston B, Thompson MC, et al. Fully implanted adaptive deep brain stimulation in freely moving essential tremor patients. J Neural Eng. 2020;17:056026. doi: 10.1088/1741-2552/abb416. [DOI] [PubMed] [Google Scholar]
- 50.Molnar GF, Sailer A, Gunraj CA, et al. Changes in cortical excitability with thalamic deep brain stimulation. Neurology. 2005;64:1913–1919. doi: 10.1212/01.WNL.0000163985.89444.DD. [DOI] [PubMed] [Google Scholar]
- 51.Parazzini M, Rossi E, Rossi L, et al. Evaluation of the current density in the brainstem during transcranial direct current stimulation with extra-cephalic reference electrode. Clin Neurophysiol. 2013;124:1039–1040. doi: 10.1016/j.clinph.2012.09.021. [DOI] [PubMed] [Google Scholar]
- 52.Parazzini M, Rossi E, Ferrucci R, et al. Modelling the electric field and the current density generated by cerebellar transcranial DC stimulation in humans. Clin Neurophysiol. 2014;125:577–584. doi: 10.1016/j.clinph.2013.09.039. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data are within the article. Requests for anonymized data should be sent to M.L. Welter at the Paris Brain Institute, 75013 Paris, France.