Abstract
Background
Cenobamate is an antiseizure medication used to treat partial-onset (focal) seizures. It is a molecule with one chiral center and a unique dual mechanism of action: enhancement of fast and slow inactivation of sodium channels with preferential inhibition of the persistent current and positive allosteric modulation of GABAA receptor-mediated ion channels.
Aims/Methods
Anticonvulsant effects of cenobamate (YKP3089; R-enantiomer), YKP3090 (S-enantiomer), and YKP1983 (racemate) were evaluated in chemically and electrically induced focal and generalized seizure models in rodents. The Genetic Absence Epilepsy Rat from Strasbourg (GAERS) model examined the effect of cenobamate on spike-wave seizures. Motor coordination was assessed with rotarod tests and minimal motor impairment exams.
Results
Early in development, cenobamate was found to have activity in focal and generalized seizure models in animals and was selected for continued development. Cenobamate prevented seizures in a dose-dependent manner, prevented seizure spread, and increased seizure threshold without potentiating seizure initiation or the development of tolerance to its anticonvulsant effects. In contrast, YKP3090 and YKP1983 were only effective against generalized tonic-clonic seizures. Cenobamate also protected mice from 6 Hz psychomotor-induced seizures. Cenobamate showed significant dose-dependent reductions in the number and cumulative duration of spike-and-wave discharges in the GAERS model.
Discussion
Cenobamate showed efficacy or efficacy signals in all animal models of epilepsy tested with a favorable risk-versus-benefit ratio, supporting its clinical use in the treatment of partial-onset (focal) seizures in adults and warranting further clinical research in generalized seizures and absence seizures.
Keywords: Epilepsy, Seizures, Animal models, Antiseizure medication, Xcopri
1. Introduction
Pharmacotherapy is the predominant initial management strategy for the treatment of epilepsy with an ultimate goal of achieving seizure freedom with minimal adverse effects [[1], [2], [3], [4], [5]]. Despite the development and availability of many new antiseizure medications (ASMs)/antiepileptic drugs in the past 30 years, some people with epilepsy still do not achieve seizure freedom with ASM treatment and/or do not tolerate ASMs due to untoward adverse events [1,3,4,6,7].
Cenobamate (also known as YKP3089, Xcopri® [SK Life Science, Inc.], Ontozry® [Angelini Pharma]; [(1R)-1-(2-Chlorophenyl)-2-(tetrazol-2-yl) ethyl] carbamate) is a new ASM approved in the US and EU for treatment of partial-onset (focal) seizures in adults. It is a molecule with one chiral center [8,9]. Cenobamate is the R-enantiomer form of the structure, YKP3090 the S-enantiomer, and YKP1983 the racemic mixture of cenobamate and YKP3090.
Cenobamate has a unique dual mechanism of action, although the precise mechanism is unknown. It enhances fast and slow inactivation of voltage-gated sodium channels and preferentially inhibits the persistent current (INaP) [[10], [11], [12]]. Cenobamate has also been shown to be a positive allosteric modulator of GABAA receptor-mediated ion channels via binding to non-benzodiazepine GABAA receptor binding sites [[11], [12], [13]].
Animal seizure and epilepsy models are used to screen, identify, and differentiate the anticonvulsant potential of new chemical entities [7]. These models mimic human seizures and have been extremely useful in predicting the clinical potential of tested compounds in patients with epilepsy [7,14,15]. Here we describe the effects of cenobamate, YKP3090, and/or YKP1983 in multiple rodent seizure and epilepsy models. The intent of this review is to show the broad spectrum of efficacy for cenobamate across these multiple animal models that formed the foundation for its drug development. Some of the studies described herein were conducted as part of the Epilepsy Therapy Screening Program (ETSP, previously called the Anticonvulsant Screening Project) through the National Institute of Neurological Disorders and Stroke (NINDS) of the National Institutes of Health (NIH) in the US.
2. Materials and methods
2.1. Animals
All studies were carried out in accordance with the National Research Council Publication “Guide for the Care and Use of Laboratory Animals” as adopted and promulgated by the NIH and were approved by the respective Institution's Animal Care and use Committee or local equivalent. Chemically and electrically induced seizure model studies, rotarod tests, and minimal motor impairment examinations were conducted at SK Biopharmaceuticals Co., LTD (New Jersey, US [now known as SK Life Science, Inc., US] and Daejeon, South Korea sites) or at the University of Utah NINDS contract facilities. The GAERS model was conducted at SynapCell SAS (France).
Eight to 16 male ICR (20–26 g) or CF-1 albino (18–26 g) mice (from Bio-Genomics, Korea or Charles River Laboratories, Inc., US) per treatment group were used to carry out chemically and electrically induced seizure model studies. Two to 24 male Sprague-Dawley (100–150 g; 275–300 g for hippocampal kindling) and CD IGS (126–150 g; International Genetic Standardization system bred) rats (from Bio-Genomics, Korea, Charles River Laboratories, Inc., and Simonsen Laboratories, Inc., US) per treatment group were used to perform chemically and electrically induced seizure model studies. Twelve male Genetic Absence Epilepsy Rat from Strasbourg (GAERS) rats (exclusively licensed for SynapCell SAS from Grenoble Institute of Neurosciences, Grenoble, France at 5 weeks old) were used in the GAERS model. Eight to 10 male ICR (20–26 g) and CF-1 (18–25 g) mice per treatment group and 6 to 12 male Sprague-Dawley (100–150 g) and CD IGS (126–150 g) rats per treatment group were used to carry out the rotarod tests and minimal motor impairment examinations. All mice and rats were housed, fed, and handled in a manner consistent with the recommendations in the "Guide for the Care and Use of Laboratory Animals" as described above. All rodents had free access to food and water and were housed under 12-h light/dark cycles in a temperature (generally between 19 °C and 24 °C) and humidity (30%–70%) controlled vivariums.
2.2. Drugs
Cenobamate was dissolved in 30% polyethylene glycol (PEG) 300 or 400 for intraperitoneal (IP) or oral (PO) administration. When assessing the enantiomers, cenobamate, YKP3090, and YKP1983 were suspended in 0.5% methylcellulose for IP or PO administration for NINDS studies. The specific vehicle for each study is detailed in Table 1. Dosing volumes for cenobamate, YKP3090, and YKP1983 ranged from 4 to 5 mL/kg for rats (Sprague-Dawley, CD IGS) and 10 mL/kg for mice (ICR, CF-1 albino). The vehicle for phenytoin, ethosuximide, and diazepam was 30% PEG 400. Valproic acid was dissolved in 30% PEG 400, distilled water or 0.9% saline. Carbamazepine was dissolved in 30% PEG 400 or 0.5% methylcellulose. Pentylenetetrazol (PTZ; Metrazol) was dissolved in distilled water or 0.9% saline. Intravenous Metrazol was dissolved in heparinized saline. Bicuculline was dissolved in warmed 0.1 N HCl and/or 0.9% saline. Picrotoxin was dissolved in 0.9% saline.
Table 1.
Experimental design of the animal antiseizure models.
| Animal Model | Study | Species | Route | Drugs | Pretreatment | Test | n/group | Observation Interval | Endpoints |
|---|---|---|---|---|---|---|---|---|---|
| MES | NINDS | CF-1 Mice | IP | 0.5% MC, CBM; YKP3090; YKP1983 | 0.25 h | 50 mA, 60 Hz, 0.2 s corneal electrodes | 8 | Within 5 s of MES | Full tonic extension of hindlimbs |
| A | ICR Mice | IP/PO | 30%PEG400, CBM, PHT | 0.5 h IP 1.0 h PO |
50 mA, 60 Hz, 0.2 s corneal electrodes | 8 | Within 5 s of MES | Full tonic extension of hindlimbs | |
| B | CF-1 Mice | IP | 30%PEG400, CBM, VPA | 0.5 h | 50 mA, 60 Hz, 0.2 s corneal electrodes | 8–10 | Within 5 s of MES | Full tonic extension of hindlimbs | |
| NINDS | SD Rats | IP/PO | 0.5% MC, CBM, YKP3090; YKP1983 | 4, 0.5, 2 h IP 1 h PO |
150 mA, 60 Hz, 0.2 s corneal electrodes | 8 | Within 5 s of MES | Full extension of tonic hindlimbs | |
| C | SD Rats | PO | 30%PEG400, CBM, CBZ | 1 h | 150 mA, 60 Hz, 0.2 s corneal electrodes | 8 | Within 5 s of MES | Full extension of tonic hindlimbs | |
| D | SD Rats | PO | 0.5% MC, CBM (3 batches), CBZ | 1 h | 180 mA, 60 Hz, 0.2 s corneal electrodes | 8 | Within 5 s of MES | Full extension of tonic hindlimbs | |
| MES tolerance | E | CF-1 Mice | IP | 30%PEG400, CBM | 4 days saline → CBM → 0.5 h 5 days CBM →0.5 h |
50 mA, 60 Hz, 0.2 s corneal electrodes | 8 | Within 5 s of MES | Full extension of tonic hindlimbs |
| 6 Hz | NINDS | CF-1 Mice | IP | 0.5% MC, CBM | 0.25 h | 22, 32, or 44 mA, 60 Hz, 0.2 s corneal electrodes | 8 | NA | Minimal clonic seizures with stereotyped automastic behaviors |
| Hippocampal kindling | NINDS | SD rat with bipolar electrodes in ventral hippocampus | IP | 0.5% MC, CBM; YKP3090; YKP1983 | Rats kindled to Stage 5 with 5 stimulus days (2 days apart) of 50 Hz 10 s 1 ms biphasic 200 μA every 30 min for 6 h On treatment day: 15 min |
20 μA + 10 μA every 1–2 min until after discharge duration | 6 | 0, 0.25, 0.75, 1.25, 1.75, 2.25 h | Racine seizure score S1-5, after discharge duration |
| After-discharge threshold | ADT | SD Rat with bipolar electrodes in ventral hippocampus | IP | 0.5% MC, CBM | Rats kindled to Stage 5 with 5 stimulus days (2 days apart) of 50 Hz 10 s 1 ms biphasic 200 μA every 30 min for 6 h On treatment day: 15 min |
20 μA + 10 μA every 1–2 min until after discharge duration | 6 | 0, 0.25, 1, 2 4 h | Racine seizure score S1-5, after discharged threshold, after discharge duration |
| PTZ | NINDS | CF-1 Mice | IP/PO | 0.5% MC, CBM, YKP3090; YKP1983 | 0.25 h | 85 mg/kg PTZ SC | 8 | 30 min | Repeated clonic seizures (>3 s) |
| F | ICR Mice | IP/PO | 30%PEG400, CBM, ESM | 0.5 h | 95.2 mg/kg PTZ SC | 8 | 30 min | Generalized clonus; repeated clonic seizures (>3 s) | |
| G | CF-1 Mice | IP | 30%PEG400, CBM, VPA | 0.5 h | 85 mg/kg PTZ SC | 8–10 | 30 min | Generalized clonus; repeated clonic seizures (>3 s); tonic extension | |
| NINDS | SD Rats | IP/PO | 0.5% MC, CBM, YKP3090, YKP1983 | 4, 0.5, 0.5 h IP 1 h PO |
56.4 mg/kg (Simonsen); 68 mg/kg (Charles River) PTZ SC | 8 | 30 min | Repeated clonic seizures (>3 s); tonic extension | |
| H | SD Rats | PO | 30%PEG400, CBM | 1 h | 105.8 mg/kg PTZ SC | 8 | 30 min | Generalized clonus; repeated clonic seizures (>3 s) | |
| I | SD Rats | PO | 30%PEG400, CBM, VPA | 1 h | 70 mg/kg PTZ SC | 4–8 | 30 min | Generalized clonus; repeated clonic seizures (>3 s) | |
| IV PTZ | NINDS | CF-1 Mice | IP | 0.5% MC, CBM, YKP3090, YKP1983 | 0.25 h | 0.34 mL/min 0.5% Metrazol IV in heparinized saline | 10 | NA | Time (s) first twitch; time (s) onset of sustained clonus |
| Bic | NINDS | CF-1 Mice | IP | 0.5% MC, CBM | 0.25 h | 2.7 mg/kg Bic SC | 8 | 30 min | Repeated clonic seizures (>3 s) |
| J | ICR Mice | IP | 30%PEG400, CBM, VPA | 0.5 h | 3.4 mg/kg Bic SC | 8 | 30 min | Generalized clonus; repeated clonic seizures (>3 s) | |
| K | CF-1 Mice | IP | 30%PEG400, CBM, DZP | 0.5 h | 3.5 mg/kg Bic SC | 7–10 | 30 min | Clonic seizures (hopping; LORR >3 s) | |
| Pic | NINDS | CF-1 Mice | IP | 0.5% MC, CBM | 0.25 h | 2.5 mg/kg Pic SC | 8 | 30 min | Repeated clonic seizures (>3 s) |
| L | ICR Mice | IP | 30%PEG400, CBM, VPA | 0.5 h | 4.5 mg/kg Pic SC | 8 | 45 min | Generalized clonus; repeated clonic seizures (>3 s) | |
| GAERS | M | GAERS Rats with 4 mono-polar electrodes frontal and parietal cortices | IP | 30%PEG400, CBM, VPA | 10 min | 1 h habituation; 20-min EEG baseline; 10 min post dose |
9 | 10 min–90 min post-dose | Number and cumulated duration of SWDs |
ADT, afterdischarge threshold; CBM, cenobamate; CBZ, carbamazepine; DZP, diazepam; EEG, electroencephalogram; ESM, ethosuximide; GAERS, Genetic Absence Epilepsy Rate from Strasbourg; h, hours; IP, intraperitoneal; IV, intravenous; LORR, loss of righting reflex; MC; methylcellulose; MES, maximal electroshock seizure; NA, not available. NINDS, National Institute of Neurological Disorders and Stroke; PEG, polyethylene glycol; PHT, phenytoin; PO, oral; PTZ, pentylenetetrazol/metrazole; s, seconds; SC, subcutaneous; SD, Sprague Dawley; SWD, spike-and-wave discharge; VPA, valproic acid/valproate.
2.3. Animal seizure models
The objective of the chemically and electrically induced seizure model studies was to assess the anticonvulsant effects of cenobamate, YKP3090, and/or YKP1983 in models of partial-onset (focal) seizures and generalized seizures. A table of the experimental designs of all of the seizure models is provided (Table 1). Please note that during the drug development of cenobamate, older seizure terminology was utilized, and it is not appropriate to map these terms to the newer International League Against Epilepsy terminology used to classify human seizures [16] after the fact. This could be considered a limitation of the animal models included herein.
2.4. Electrically induced seizure models
The maximal electroshock seizure (MES) test is a model of human generalized tonic-clonic seizures and provides an assessment of a drug's ability to prevent seizure spread [15,[17], [18], [19], [20], [21]]. MES testing was conducted with a 50 mA, 60 Hz current delivered for 0.2 s to mice (8–10/group). In rats (8/group), testing was conducted with a 150 mA or 180 mA, 60 Hz current delivered for 0.2 s, with one study comparing the activity of three different batches of cenobamate. Vehicle or compounds were injected either IP or PO, and then 0.5 or 1.0 h later, electric current was delivered via corneal electrodes. Animals were observed for approximately 5 s for full hindlimb extension or tonic seizures.
In the MES tolerance test (8 mice/group), cenobamate was administered for 5 consecutive days at a dose of 7.5 mg/kg IP (based on a previously determined MES ED50) in the cenobamate group, and vehicle was administered for 4 consecutive days followed by 1 dose of cenobamate 7.5 mg/kg on the fifth day in the control group. Thirty minutes after the last IP administration, electric current (50 mA; 60 Hz, 0.2 s) was administered via corneal electrodes and mice were observed for tonic seizures for approximately 5 s.
The 6 Hz psychomotor seizure test using convulsive currents of 22, 32, and 44 mA (6 Hz, 3 s) was performed to assess the effect of treatment in a drug-resistant psychomotor (focal) seizure model [20,22,23]. Mice (8/group) were injected IP with vehicle or compound 15 min prior to testing. A drop of anesthetic/electrolyte solution (0.5% tetracaine HCl in 0.9% saline) was applied to the eyes of each animal prior to electric current delivery. A mouse was protected if no seizure with a minimal clonic phase followed by stereotyped, automatistic behaviors was observed.
The effect of treatment on focal seizure duration and severity was determined in the hippocampal kindled rat model of focal epilepsy by measuring the effect of treatment on Racine seizure score and electrographic afterdischarge threshold and duration [15,20,21]. Briefly, a bipolar electrode was stereotaxically placed into the ventral hippocampus (AP -3.6, ML -4.9, VD -5.0 from dura, incisor bar +5.0) of adult male Sprague-Dawley rats (275–300 g), under ketamine-xylazine anesthesia [24]. After a one-week recovery period, animals were kindled to a Stage 5 behavioral seizure using a stimulus consisting of a 50 Hz, 10 s train of 1 ms biphasic 200 μA pulses delivered every 30 min for 6 h (12 stimulations per day) on alternating days for a total of 60 stimulations (5 stimulus days) [25]. Drug testing was initiated after a one-week, stimulus-free period. The stability of the behavioral seizure stage and afterdischarge duration was assessed by delivering 2–3 suprathreshold stimulations every 30 min prior to drug treatment. Following the last control block, a single IP dose of the compound was administered; 15 min later, each rat (n = 8) was stimulated every 30 min for 2 h. After each stimulation, individual seizure scores and afterdischarge durations were recorded. Seizures were scored according to the following Racine criteria: Stage 1 - mouth and facial clonus; Stage 2 - Stage 1 plus head nodding; Stage 3 - Stage 2 plus forelimb clonus; Stage 4 - Stage 3 plus rearing; Stage 5 - Stage 4 plus repeated rearing and falling over [26]. It should be noted that Racine scores were recorded during the in-life of the experiment and therefore could not be adjusted post-hoc to accommodate subsequent modifications of Racine criteria. In the follow-up ADT study, hippocampal kindled rats prepared as above were tested for individual afterdischarge thresholds. An initial stimulation was conducted at an intensity of 20 μA. Then the stimulus intensity was increased in 10 μA increments every 1–2 min until an afterdischarge was elicited. Fifteen minutes after the baseline threshold determination, the compound was administered IP. The individual rat afterdischarge threshold was then re-determined at 0.25-, 1-, 2-, and 4-h post-injection. Seizure scores and afterdischarge durations were noted along with the afterdischarge durations for each rat at each timepoint.
2.5. Chemically induced seizure models
The subcutaneous (SC) PTZ test was used to evaluate the effects on generalized or repeated clonic seizures [15,21,27,28]. Mice (8/group) were administered vehicle or compound (IP or PO). Then 30 min later, they were administered PTZ at 95.2 mg/kg SC and immediately observed for the next 30 min for signs of generalized clonus (clonic seizures with the loss of righting reflex) or repeated clonic seizures (contraction of fore- and/or hindlimbs >3 s duration). In another study, mice (8–10/group) were injected with vehicle or compound IP. Then 30 min later, they were administered PTZ at 85 mg/kg SC and immediately observed for clonic seizures (loss of righting reflex >3 s) or tonic extension of the hindlimbs. In the NINDS study, mice (8/group) were administered 85 mg/kg PTZ SC. Rats (8/group) were administered vehicle or compound (PO). Then 60 min later, they were administered PTZ at 105.8 mg/kg SC and immediately observed for the next 30 min for signs of generalized clonus or repeated clonic seizures (>3 s duration). Rats (8/group) were administered vehicle or compound (PO). Then 60 min later, they were administered PTZ at 105.8 mg/kg SC and immediately observed for the next 30 min for signs of generalized clonus or repeated clonic seizures (>3 s duration). In another study, rats (4–8/group) were administered vehicle or compound (PO). Sixty minutes later, they were administered PTZ at 70 mg/kg SC and immediately observed for the next 30 min for signs of generalized clonus or repeated clonic seizures (>3 s duration). In the NINDS Study, rats (8/group) were administered PTZ SC at either 56.4 mg/kg (Simonsen source) or 68 mg/kg (Charles River source) prior to being observed for 30 min. Rats were protected if no clonic seizures >3 s duration were noted.
The SC bicuculline test assessed for effects in clonic/tonic (generalized) seizures [19,21,29]. Vehicle or compound was administered to mice (8/group) IP, and then 30 min later, they were injected with 3.43 mg/kg bicuculline SC and immediately observed for the next 30 min for signs of generalized clonus or repeated clonic seizures. In another study, vehicle or compound was administered to mice (7–10/group) IP. Then 30 min later they were injected with 3.5 mg/kg bicuculline SC and immediately observed for the next 30 min for clonic seizures (hopping with loss of righting reflex for >3 s) or tonic seizures (extension of the hindlimbs). In the NINDS study, mice (8/group) were administered 2.7 mg/kg bicuculline SC and observed for 30 min.
The SC picrotoxin test determined effects in clonic (generalized) seizures [21,29]. Vehicle or compound was administered to mice (8/group) IP, and then 30 min later, they were injected with 4.47 mg/kg picrotoxin SC and immediately observed for the next 30 min for signs of generalized clonus or repeated clonic seizures. In the NINDS study, mice (8/group) were administered 2.5 mg/kg picrotoxin SC and observed for 30 min.
The effect of treatment on seizure threshold was determined using the intravenous (IV) PTZ seizure threshold test [21]. The timed intravenous infusion (0.34 mL/min) of 0.5% Metrazol in heparinized saline to mice is used to identify those compounds that lower seizure threshold and therefore may be proconvulsant [30]. Vehicle or compound was administered IP (10 mice/group) and then at the time of peak effect, the Metrazol solution was infused via the tail vein. Then the time to "first twitch" of the whole body in seconds and the time to "sustained clonus" of forelimbs in seconds were recorded. Results obtained with groups of 10 mice were then converted to mg/kg of Metrazol required to induce each endpoint.
2.6. GAERS model
The objective of the GAERS study was to assess the effects of cenobamate on spike-and-wave seizures in an animal model of absence seizures. The GAERS model was performed based on the methods described in Depaulis et al., 2016. A total of 12 GAERS rats under general anesthesia were stereotaxically implanted with 4 monopolar electrodes on both sides of the frontal and parietal lobes to allow electroencephalograms (EEG) recording. After 1 week of recovery, GAERS rats were tested in a 1-h EEG session and 10 rats with sufficient signal-to-noise ratio for detection of spike-and-wave discharges (SWDs) were then randomized in a crossover design to receive 5, 10, 20, or 30 mg/kg cenobamate; 150 or 200 mg/kg valproate (0.9% saline) and vehicle (30% PEG 300) IP (5 mL/kg injection volume) with at least 3-day washouts. EEGs were performed on freely moving rats for 20 min pre-dose and 80 min after compound administration (starting 10 min post-dose). Coded EEGs were analyzed offline by blinded experts to identify SWDs. An SWD starts and ends abruptly lasting 17–25 s and is associated with a behavioral arrest during the time of the discharge. The presence and duration of SWDs were quantified in 20-min intervals. After analysis, one animal out of 10 was omitted from analysis as the baseline number of SWDs was below the criteria of 12.
2.7. Rotarod test and minimal motor impairment
The objective of the rotarod test studies was to assess the effects of cenobamate, YKP3090, and YKP1983 on motor coordination and determine toxicity. The rotarod test was performed based on the methods described in White et al., 1998 [21] and Dunham and Miya [31]. All drugs were prepared and administered to mice or rats as described above. Mice were trained to walk on 3.2 cm diameter rotating rod (6.9–7 revolutions/minute) for two 10-min sessions at least 30 min apart. Mice (8/group/interval) were injected with compounds either IP or PO and then tested on the rotarod at 0.25-, 0.5-, 1-, 2-, and/or 4-h post-injection. Each test lasted 60 s. A mouse passed if it did not fall more than once or three times (NINDS study). Rats were trained to walk on a 7.3 cm diameter rotating rod (6.9–7 revolutions/minute) for either two 5- or 10-min training sessions at least 30 min apart. Rats (6–8/group/interval) were injected with compounds PO and then tested for 1 min at 0.5-, 1-, 2-, 4- or 5-h post-injection. A rat failed the rotarod if it fell more than once in 1 min.
Minimal motor impairment (MMI; NINDS study) was assessed in rats by visual observation of gait, stance, placing response, exploratory behavior, and muscle tone [21]. Rats (2–24/group/interval) were observed for MMI at 0.25-, 0.5-, 1-, 2- and 4-h post IP or PO administration of cenobamate, YKP3090 or YKP1983. The experimental designs of these neurotoxic tests are outlined in Table 2.
Table 2.
Experimental design of the neurotoxicity animal models.
| Animal Model | Study | Species | Route | Drugs | Training | Test | n/group | Observation Interval | Endpoints |
|---|---|---|---|---|---|---|---|---|---|
| Rotarod | NINDS | CF-1 Mice | IP | 0.5% MC; CBM, YKP3090; YKP1983 | None | 1 inch diameter rotating rod 6 rpm | 8 | 0.25 h | Fail if fell off 3 times in 1 min |
| O | ICR Mice | IP/PO | 30% PEG400, CBM | 2 10-min sessions at least 30 min apart | 3.2 cm diameter rod 7 rpm | 8 | 0.5, 1, 2, 4 h | Fail if fell off more than 1 time in 1 min | |
| P | CF-1 Mice | IP | 30% PEG400, CBM | 2 10-min sessions at least 30 min apart | 3.2 cm diameter rod 6.9 rpm | 8 | 0.25, 0.5, 1, 2 h | Fail if fell off more than 1 time in 1 min | |
| Q | SD Rats | PO | 30% PEG400, CBM | 2 10-min sessions at least 30 min apart | 7.3 cm diameter rod 7 rpm | 8 | 0.5, 1, 2, 4 h | Fail if fell off more than 1 time in 1 min | |
| R | SD Rats | PO | 0.5% MC, CBM | 2 5-min sessions at least 30 min apart | 3.2 cm diameter rod 6.9 rpm | 6 | 0.5, 1, 2, 4, 5 h | Fail if fell off more than 1 time in 1 min | |
| S | CD IGS Rats | PO | 0.5% MC, CBM (3 batches) | 2 5-min sessions at least 30 min apart | 7 cm diameter rod 6.9 rpm | 6 | 0.5, 1, 2, 4, 5 h | Fail if fell off more than 1 time in 1 min | |
| Minimum Motor Impairment | NINDS | SD Rat | IP/PO | 0.5% MC; CBM, YKP3090; YKP1983 | NA | NA | 2–24 | 0.25, 0.5, 1, 2, 4 h | Overt evidence of ataxia, abnormal gait and stance |
CBM, cenobamate; h, hours; IP, intraperitoneal; MC, methylcellulose; NA, not available; NINDS, National Institute of Neurological Disorders and Stroke; PEG, polyethylene glycol; PO, oral; SD, Sprague Dawley.
2.8. Data analysis
Median effective dose (ED50, the dose which effectively protected 50% of the animals from seizures) or time to seizure event for cenobamate, YKP3090, and YKP1983 was determined in partial-onset (focal) and generalized seizure models, including MES and 6 Hz tests. The median ED50 for cenobamate was also determined in the following chemically induced seizure models: SC bicuculline test and SC picrotoxin test. The Toxic Dose50 (TD50) is defined as the dose that produced minimal impairment as estimated by rotarod performance (mice or rats) or minimal motor impairment (rats). TD50 values were determined for cenobamate (with one study assessing three different batches of cenobamate), YKP3090, and YKP1983.
The median ED50 and TD50 values with corresponding 95% confidence intervals, if applicable, were calculated by a computer probit analysis program according to the Litchfield and Wilcoxon or Finney methods [32,33]. The protective index (PI) ratio was calculated by dividing TD50 value by ED50 value. The larger the PI, the more likely the ASM will provide seizure protection without behavioral toxicity [12,21,34]. Data for hippocampal kindling, the timed IV PTZ threshold test, and GAERS models were expressed as mean ± standard error of the mean (SEM). Hippocampal kindling data (NINDS, ADT) were analyzed with a nonparametric Mann-Whitney U test (seizure scores) and Student t-tests (afterdischarge durations and/or afterdischarge thresholds) and compared to Time 0. Seizure scores are interval data where normality is not generally met as kindled animals are pre-selected according to their fully kindled state. The timed IV PTZ threshold test data were analyzed using a Student t-test. Afterdischarge durations and PTZ threshold doses are continuous data and generally meet parametric statistic criteria of normality, homoscedasticity, independence, and low outlier rate. Time-course of number and cumulative duration of SWDs in the GAERS model were analyzed by multiple nonparametric Friedman's tests, followed by Dunn's multiple comparisons tests versus baseline, and vehicle with significance defined as P < 0.05.
3. Results
3.1. Murine models
3.1.1. Chemically and electrically induced seizures
Cenobamate, YKP3090, and YKP1983 administered IP were all effective against SC PTZ- and MES-induced seizures in mice. The median ED50 values were 28.5, 56.4, and 35.9 mg/kg IP for SC PTZ-induced seizures and 9.8, 38.2, and 15.8 mg/kg IP for MES-induced seizures, respectively. Cenobamate administered as an IP dose was also effective against SC picrotoxin (median ED50 of 34.5 mg/kg IP) but not SC bicuculline-induced (median ED50 of >70 mg/kg IP) seizures. After IP doses, cenobamate was effective against 6 Hz-induced seizures at all three tested stimulus intensities (22, 32, and 44 mA); median ED50 values of 11.0, 17.9, and 16.5 mg/kg IP with PIs of 5.3, 3.2, and 3.5 were determined, respectively (Table 3).
Table 3.
Chemically and electrically induced seizure studies in mice with IP administration of cenobamate, YKP3090, and YKP1983 (NINDS study, n = 8–16/group).
| Type of Seizure Model | Seizure Model | Parameter | Cenobamate | YKP3090 | YKP1983 | |
|---|---|---|---|---|---|---|
| NA | Rotarod test | TD50 mg/kg (95% CI) | 58.0 (39.5–74.1) | 143 (123–164) | 92.5 (80.3–114) | |
| Chemically induced | SC PTZ 85 mg/kg | ED50 mg/kg (95% CI) | 28.5 (19.6–42.3) | 56.4 (43.0–71.3) | 35.9 (26.9–46.6) | |
| PIa | 2.0 | 2.5 | 2.6 | |||
| SC bicuculline 2.7 mg/kg | ED50 mg/kg (95% CI) | >70 | NT | NT | ||
| PIa | <0.8 | NA | NA | |||
| SC picrotoxin 2.5 mg/kg | ED50 mg/kg (95% CI) | 34.5 (25.9–48.8) | NT | NT | ||
| PIa | 1.7 | NA | NA | |||
| Electrically induced | 6 Hz | 22 mA | ED50 mg/kg (95% CI) | 11.0 (6.9–15.4) | NT | NT |
| PIa | 5.3 | NA | NA | |||
| 32 mA | ED50 mg/kg (95% CI) | 17.9 (14.9–21.1) | NT | NT | ||
| PIa | 3.2 | NA | NA | |||
| 44 mA | ED50 mg/kg (95% CI) | 16.5 (11.6–22.6) | NT | NT | ||
| PIa | 3.5 | NA | NA | |||
| MES | ED50 mg/kg (95% CI) | 9.8 (7.8–12.9) | 38.2 (25.4–57.2) | 15.8 (14.4–17.9) | ||
| PIa | 5.9 | 3.7 | 5.9 | |||
CI, confidence interval; ED50, median effective dose; IP, intraperitoneal; MES, maximal electroshock seizures; NA, not applicable/available; NINDS, National Institute of Neurological Disorders and Stroke; NT, not tested; PI, protective index; PTZ, pentylenetetrazol; SC, subcutaneous; TD50, median neurotoxic dose.
Protective index = TD50/ED50. TD50 based on rotarod test.
In the timed IV PTZ test (0.25-h post dose) in mice, IP doses of cenobamate significantly elevated the seizure threshold at the TD50 dose of 58 mg/kg IP (i.e., cenobamate significantly increased the mg/kg of PTZ required to reach first twitch and clonus). There were no effects on IV PTZ seizure threshold at the MES ED50 (i.e., 10 mg/kg IP). YKP3090 significantly elevated the seizure threshold at the MES ED50 dose of 38 mg/kg IP and at the TD50 of 143 mg/kg IP. YKP1983 also elevated the seizure threshold at the MES ED50 dose of 16 mg/kg IP and TD50 dose of 93 mg/kg IP (Table 4).
Table 4.
Timed (0.25 h post dose) IV PTZ study in mice with IP administration of cenobamate, YKP3090, and YKP1983 (NINDS and ADT study, n = 10/group).
| Test Compound | Dose (mg/kg) | Approximate equivalent (mg/kg) | Time to First Twitch (s) | First Twitch (mg/kg of PTZ) | Time to Clonus (s) | Clonus (mg/kg of PTZ) |
|---|---|---|---|---|---|---|
| Control | 0 | – | 35.0 ± 1.2 | 35.3 ± 1.3 | 40.6 ± 1.4 | 40.9 ± 1.3 |
| Cenobamate | 10 | MES ED50 | 35.0 ± 2.2 | 36.2 ± 2.4 | 42.1 ± 2.4 | 43.6 ± 2.7 |
| 58 | TD50 | 46.3 ± 1.9* | 47.4 ± 1.9* | 64.1 ± 2.2* | 65.8 ± 2.8* | |
| Control | 0 | – | NA | 30.0 ± 1.8 | NA | 34.3 ± 2.4 |
| YKP3090 | 38 | MES ED50 | NA | 45.9 ± 2.7* | NA | 60.0 ± 6.1* |
| 143 | TD50 | NA | 69.9 ± 4.7* | NA | 112.3 ± 9.3* | |
| Control | 0 | – | NA | 31.9 ± 0.6 | NA | 38.6 ± 2.3 |
| YKP1983 | 16 | MES ED50 | NA | 35.8 ± 1.6* | NA | 42.7 ± 1.8 |
| 93 | TD50 | NA | 62.5 ± 2.8* | NA | 74.4 ± 3.4* |
ADT, afterdischarge threshold; IP, intraperitoneal; IV, intravenous; NA, not available; NINDS, National Institute of Neurological Disorders and Stroke; PTZ, pentylenetetrazol; s, seconds; SEM, standard error of the mean.
Data reported as mean ± standard error of the mean.
*Indicating statistically significant differences (P < 0.05) compared to control.
Cenobamate displayed a broad spectrum of antiseizure efficacy in comparison to select prototype ASMs [12] as it was active in seizure models predictive of focal and generalized seizures at doses devoid of behavioral toxicity in mice. YKP3090 and YKP1983 were also active in seizure models predictive of generalized seizures at doses devoid of behavioral toxicity, although they were not tested in the full complement of seizure tests in mice.
Taken as a whole, cenobamate demonstrated activity in multiple chemically and electrically induced seizure models in mice (Table 5). IP and PO doses of cenobamate inhibited SC PTZ-induced seizures (median ED50 of 3.8–28.5 mg/kg IP and 7.1 mg/kg PO) in a dose-dependent manner. Seizure inhibition following IP administered cenobamate was also noted in mice tested in SC bicuculline-induced (median ED50 of 17.3–35.4 mg/kg IP in two studies) and SC picrotoxin-induced (median ED50 of 23.2–34.5 mg/kg IP in two studies) seizure models. IP and PO doses of cenobamate protected against MES-induced seizures in a dose-dependent manner (median ED50 of 4.2–9.8 mg/kg IP and 3.3 mg/kg PO). In the NINDS MES study, cenobamate after IP dosing had a median ED50 of 9.8 mg/kg IP with a PI of 5.9. In the MES tolerance test, there was no apparent difference in protection against MES-induced seizures between the control group (single dose of cenobamate 7.5 mg/kg IP), where 4 of 8 mice were protected, and the cenobamate group (repeated cenobamate 7.5 mg/kg IP dosing for 5 consecutive days), where 3 of 8 mice were protected.
Table 5.
Anticonvulsant activity of cenobamate in mice (multiple studies).
| Type of Seizure Model | Seizure Model (STUDY) | Drug, Route & Dose | N per dose group | ED50 mg/kg (95% CI) | TD50 mg/kg (95% CI) | Protective Indexa | Additional Findings | |
|---|---|---|---|---|---|---|---|---|
| Chemically induced | SC PTZ 85 mg/kg (NINDS) | CBM IP 12, 22, 40, 95 mg/kg | 8 | 28.5 (19.6–42.3) | 58.0 (39.5–74.1) | 2.0 | Inhibition | |
| SC PTZ 95.2 mg/kg (F) | CBM IP 3, 5, 8, 12 mg/kg | 8 | 3.8 (2.3–6.2) | NT | NA | DD inhibition | ||
| ESM IP 70, 80, 90, 100 mg/kg | 8 | 96.2 (77.3–96.1) | NT | NA | DD inhibition | |||
| CBM PO 5, 7, 10 mg/kg | 8 | 7.1 (5.3–9.4) | NT | NA | DD inhibition | |||
| ESM PO 200, 250, 300 mg/kg | 8 | 210.6 (181–245) | NT | NA | DD inhibition | |||
| SC PTZ 85 mg/kg (G) | CBM IP 10, 14, 17, 30 mg/kg | 8–10 | 14.8 (12.6–17.5) | NT | NA | DD inhibition | ||
| VPA IP 150 mg/kg | 8 | NA | NT | NA | 2/8 mice protected | |||
| SC bicuculline 2.7 mg/kg (NINDS) | CBM IP 30, 70 mg/kg | 8 | >70 (−) | 58.0 (39.5–74.1) | <0.8 | No inhibition | ||
| SC bicuculline 3.4 mg/kg (J) | CBM IP 10, 20, 30 mg/kg | 8 | 17.3 (10.9–27.8) | NT | NA | DD inhibition | ||
| VPM IP 400, 450, 500 mg/kg | 8 | 468.7 (442–497) | ||||||
| SC bicuculline 3.5 mg/kg (K) | CBM IP 10, 20, 30, 40, 50 mg/kg | 10 | 35.4 (−) | NT | NA | DD inhibition from 10 to 40 mg/kg | ||
| DZP IP 5 mg/kg | 7 | NA | NT | NA | 7/7 mice protected | |||
| SC picrotoxin 2.5 mg/kg (NINDS) | CBM IP 25, 30, 35, 70 mg/kg | 8 | 34.5 (25.9–48.8) | 58.0 (39.5–74.1) | 1.7 | Inhibition | ||
| SC picrotoxin 4.5 mg/kg (L) | CBM IP 10, 20, 30 mg/kg | 8 | 23.2 (14.9–36.2) | NT | NA | DD inhibition | ||
| VPA IP 200, 300, 350, 400 mg/kg | 8 | 341 (307–380) | ||||||
| Electrically induced | 6 Hz (NINDS) | 22 mA | CBM IP 5.4, 10, 15, 20 mg/kg | 8 | 11.0 (6.9–15.4) | 58.0 (39.5–74.1) | 5.3 | – |
| 32 mA | CBM IP 10, 17, 21, 25 mg/kg | 8–16 | 17.9 (14.9–21.1) | 3.2 | – | |||
| 44 mA | CBM IP 5.4, 10, 20, 25 mg/kg | 8 | 16.5 (11.6–22.6) | 3.5 | – | |||
| MES (NINDS) | CBM IP 6, 8, 15, 23 mg/kg | 8 | 9.8 (7.8–12.9) | 58.0 (39.5–74.1) | 5.9 | Inhibition | ||
| MES (A) | CBM IP 3, 8, 12, 15 mg/kg | 8 | 4.2 (2.7–6.7) | NT | NA | DD inhibition | ||
| PHT IP 1,2, 5 mg/kg | 8 | 2.8 (1.5–5.3) | NT | NA | DD inhibition | |||
| CBM PO 2, 4, 7, 10 mg/kg | 8 | 3.3 (2.2–5.0) | NT | NA | DD inhibition | |||
| PHT PO 1,2, 5 mg/kg | 8 | 6.8 (4.7–9.6) | NT | NA | DD inhibition | |||
| MES (B) | IP 3, 5, 7.5, 10 mg/kg | 10 | 7.0 (5.6–8.6) | NT | NA | DD inhibition | ||
| VPA IP 275 mg/kg | 10 | NA | NT | NA | 6/10 mice protected | |||
CBM, cenobamate; CI, confidence interval; DD, dose-dependent; DZP, diazepam; ED50, median effective dose; ESM, ethosuximide; IP, intraperitoneal; MES, maximal electroshock seizures; NA, not applicable/available; NINDS, National Institute of Neurological Disorders and Stroke; NT, not tested; PHT, phenytoin; PO, oral; PTZ, pentylenetetrazol; SC, subcutaneous; TD50, median neurotoxic dose; VPA, valproic acid/valproate.
aProtective index calculation TD50/ED50. TD50 based on rotarod test.
3.2. Rotarod test
The median TD50 values for cenobamate, YKP3090, and YKP1983 in mice are shown in Table 6. The median TD50 for cenobamate ranged from 52 to 58 mg/kg following IP dosing and 85.6 mg/kg following PO dosing. The peak activity times in cenobamate-treated mice were 0.5- and 2-h post-dose after IP and PO doses, respectively. The median TD50 for YKP3090 was 143 mg/kg IP and for YKP1983 it was 92.5 mg/kg IP with peak activity times of 0.25-h post-dose for both compounds.
Table 6.
Effect of cenobamate, YKP3090, and YKP1983 on the rotarod test in mice (multiple studies).
| Test Compound (STUDY) | Route & Dose | N per dose group | Time of Test (post-dose in h) | TD50 mg/kg (95% CI) |
|---|---|---|---|---|
| Cenobamate (O) | PO 80, 90, 100, 120 mg/kg | 8 | 2 | 85.6 (78.7–93.0) |
| IP 45, 50, 60, 80 mg/kg | 8 | 0.5 | 52.0 (46.0–58.7) | |
| Cenobamate (P) | IP 50, 60, 75 mg/kg | 8 | 0.25 | 57.9 (41.7–80.4) |
| 8 | 1 | 55.7 (18.1–171.9) | ||
| Cenobamate (NINDS) | IP 40, 55, 75, 95 mg/kg | 8 | 0.25 | 58.0 (39.5–74.1) |
| YKP3090 (NINDS) | IP 90, 110, 135, 180, 220 mg/kg | 8 | 0.25 | 143 (123–164) |
| YKP1983 (NINDS) | IP 50, 60, 70, 80, 110, 170 mg/kg | 8 | 0.25 | 92.5 (80.3–114) |
CI, confidence interval; h, hours; IP, intraperitoneal; NINDS, National Institute of Neurological Disorders and Stroke; PO, oral; TD50, median neurotoxic dose.
3.3. Rat models
3.3.1. Chemically and electrically induced seizures
As summarized in Table 7, cenobamate, YKP3090, and YKP1983 were effective after IP dosing in rats against SC PTZ (median ED50 of 13.6, 16.7, and 19.3 mg/kg IP, respectively) and MES-induced seizures (median ED50 of 2.9, 26.1, and 8.4 mg/kg IP, respectively). After IP dosing, cenobamate was active against hippocampal kindling-induced seizures (median ED50 of 16.4 mg/kg IP), however, YKP3090 (median ED50 of >50 mg/kg IP) and YKP1983 (inactive at 30 mg/kg IP) were not active. After PO dosing, YKP3090 was effective against SC PTZ-induced seizures (median ED50 of 8.14 mg/kg PO) while cenobamate and YKP1983 were only partially effective. Cenobamate, YKP3090, and YKP1983 were all effective after PO dosing against MES-induced seizures (median ED50 of 1.9, 11.9, and 2.9 mg/kg PO, respectively).
Table 7.
Chemically and electrically induced seizure studies in rats with PO and IP administration of cenobamate, YKP3090, and YKP1983 (NINDS study, n = 8–24/group).
| Seizure Model | Parameter | PO |
IP |
||||
|---|---|---|---|---|---|---|---|
| Cenobamate | YKP3090 | YKP1983 | Cenobamate | YKP3090 | YKP1983 | ||
| Minimum motor impairment | TD50 mg/kg (95% CI) | 50.7 (35.7–63.0) | <150 | 81.1 (61.1–112) | 38.9 (32.2–43.9) | 50.9 (33.1–67.8) | 53.3 (41.5–62.2) |
| SC PTZ 56.4 mg/kg or 68 mg/kga | ED50 mg/kg (95% CI) | Max. 40% protection at 25 | 8.14 (0.74–16.9) | Max. 33% protection at 12.5 | 13.6 (6.6–25.0) | 16.7 (14.8–18.7) | 19.3 (12.4–28.5) |
| PIb | NA | <18 | NA | 2.9 | 3.0 | 2.8 | |
| Hippocampal kindling | ED50 mg/kg (95% CI) | NT | NT | NT | 16.4 (12.7–20.2) | >50 (−) | Inactive at 30 |
| PIb | NA | NA | NA | 2.4 | <1 | NA | |
| MES | ED50 mg/kg (95% CI) | 1.9 (0.9–3.3) | 11.9 (8.5–15.0) | 2.9 (2.0–3.7) | 2.9 (1.9–3.8) | 26.1 (14.6–41.9) | 8.4 (6.9–9.8) |
| PIb | 27 | <13 | 28 | 14 | 2.0 | 6.4 | |
CI, confidence interval; ED50, median effective dose; IP, intraperitoneal; MES, maximal electroshock seizures; NA, not applicable/available; NINDS, National Institute of Neurological Disorders and Stroke; NT, not tested; PI, protective index; PO, oral; PTZ, pentylenetetrazol; SC, subcutaneous; TD50, median neurotoxic dose.
a56.4 mg/kg for rats sourced from Simonsen Laboratories, Inc. and 68 mg/kg for rats sourced from Charles River Laboratories.
bProtective index calculation TD50/ED50. TD50 based on minimal motor impairment.
In comparison to select prototype ASMs [12], cenobamate exhibited a broad spectrum of efficacy in models predictive of focal and generalized seizures in rats at doses that lacked behavioral toxicity. YKP3090 and YKP1983 were also active in these focal and generalized seizure models; however, they were not shown to be active in the rat hippocampal kindling model.
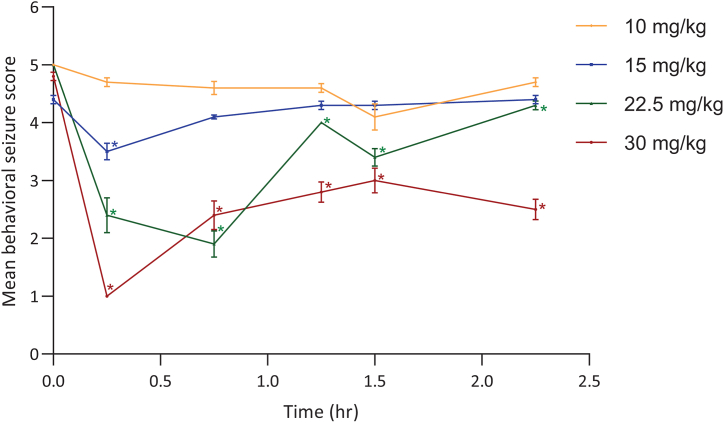
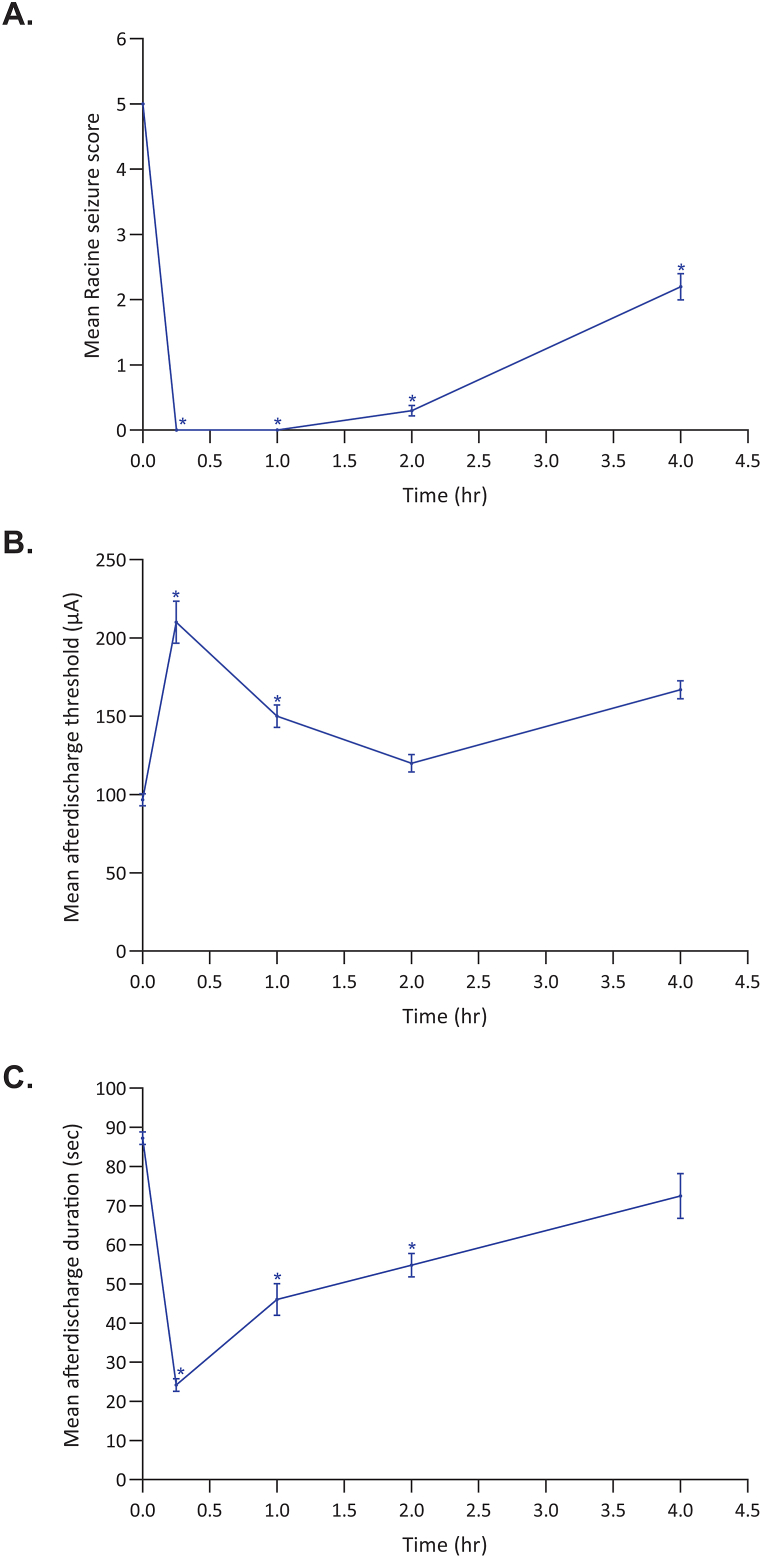
The overall activity of cenobamate across multiple chemically and electrically induced seizure models in rats is summarized in Table 8. Cenobamate inhibited SC PTZ-induced seizures following IP administration (PI of 2.9 for IP) and showed dose-dependent inhibition following PO administration (median ED50 of 8.3–20.3 mg/kg PO). In the MES studies, inhibition was seen after IP and PO administration of cenobamate (median ED50 of 2.9 mg/kg IP with a PI of 14; median ED50 of 0.4–1.9 mg/kg PO with a PI of 27; calculated based on TD50 values from minimal motor impairment). Dose-related inhibition was similar across three different cenobamate batches in a separate MES study (median ED50 of 0.3, 0.3, and 0.7 mg/kg PO with PI of 339, 347, and 143, respectively; calculated based TD50 values from rotarod performance). In the NINDS hippocampal kindling study, IP cenobamate significantly reduced the behavioral seizure score (median ED50 of 16.4 mg/kg IP) (Fig. 1) but did not affect the afterdischarge duration (data not shown). In the ADT hippocampal kindling study, cenobamate at 40 mg/kg IP significantly decreased the mean Racine seizure score at 0.25-, 1-, 2-, and 4-h post-dose (Fig. 2A); elevated the afterdischarge threshold at 0.25- and 1-h post-dose (Fig. 2B); and decreased the afterdischarge duration at 0.25-, 1-, and 2-h post-dose (Fig. 2C).
Table 8.
Anticonvulsant activity of cenobamate in rats (multiple studies).
| Type of Seizure Model | Seizure Model (STUDY) | Drug, Route & Dose | N per dose group | ED50 mg/kg (95% CI) | TD50 mg/kg (95% CI) | Protective Indexb | Additional Findings |
|---|---|---|---|---|---|---|---|
| Chemically induced | SC PTZ 56.4 mg/kg or 68 mg/kg (NINDS)a | CBM IP 4, 8, 16, 24, 32 mg/kg | 8 | 13.6 (6.6–25.0) | 38.9 (32.2–43.9) | 2.9 | Inhibition |
| CBM PO 12.5, 25, 50, 100 mg/kg | 2–10 | >100 (−) | 50.7 (35.7–63.0) | NA | Maximum 40% protection at 25 mg/kg | ||
| SC PTZ 105.8 mg/kg (H) | CBM PO 5, 10, 20 mg/kg | 8 | 8.3 (5.5–12.3) | NT | NA | DD inhibition | |
| SC PTZ 70 mg/kg (I) | CBM, PO 10, 30, 60 mg/kg | 8 | 20.3 (−) | NT | NA | DD inhibition | |
| VPA IPO 200 mg/kg | 6 | NA | NT | NA | 3/6 rats protected | ||
| Electrically induced | Hippocampal kindling (NINDS) | CBM IP 10, 15, 22.5, 30 mg/kg | 7–8 | 16.4 (12.7–20.2) | 38.9 (32.2–43.9) | 2.4 | See Fig. 2 |
| MES (NINDS) | CBM IP 1.5, 3, 4.5, 6 mg/kg | 8 | 2.9 (1.9–3.8) | 38.9 (32.2–43.9) | 14 | Inhibition | |
| CBM PO 0.75, 1.5, 3, 4.5 mg/kg | 8 | 1.9 (0.9–3.3) | 50.7 (35.7–63.0) | 27 | |||
| MES (C) | CBM PO 0.3, 0.6, 1, 3 mg/kg | 8 | 0.4 (0.3–0.8) | NT | NA | DD inhibition | |
| CBZ PO 10 mg/kg | 8 | NA | NT | NA | 6/8 rats protected | ||
| MES (D) | CBM PO 0.1, 0.3, 1, 3 mg/kg (3 batches; 1401-1401-07-001; 1401-1401-05-501; DIT040503) | 6–8 | 0.3 (−) | 101.6 (27.9–220.1) | 339c | Dose-related inhibition similar across different batches | |
| 6–8 | 0.3 (−) | 104.1 (40.0–204.4) | 347c | ||||
| 6–8 | 0.7 (−) | 100.4 (−2.0-305.9) | 143c | ||||
| CBZ PO 10 mg/kg | 8 | NA | NT | NA | 6/8 rats protected |
CBM, cenobamate; CBZ, carbamazepine; DD, dose-dependent; ED50, median effective dose; IP, intraperitoneal; MES, maximal electroshock seizures; NA/-, not applicable/available; NINDS, National Institute of Neurological Disorders and Stroke; NT, not tested; PO, oral; PTZ, pentylenetetrazol; SC, subcutaneous; TD50, median neurotoxic dose; VPA, valproic acid/valproate.
a56.4 mg/kg for rats sourced from Simonsen Laboratories, Inc. and 68 mg/kg for rats sourced from Charles River Laboratories.
bProtective index calculation TD50/ED50. TD50 based on minimal motor impairment or crotarod impairment.
Fig. 1.
Effect of IP cenobamate on mean behavioral seizure scores in hippocampal kindled rats (NINDS study, n = 7–8/group). In the hippocampal kindled rat, cenobamate was effective in significantly reducing the expression of Stage 5 Racine seizure scores with an ED50 of 16.4 mg/kg IP. Seven to 8 rats/dose were delivered supra-threshold electrical stimulation via a ventral hippocampal bipolar electrode prior to (Time 0.0) and at intervals between 15 min and 2 h:15 min post-cenobamate administration and after each stimulation, individual seizure scores were recorded. Data reported as mean ± standard error of the mean. *Indicating statistically significant differences (P < 0.05) from Time 0.0.
Fig. 2.
Effect of IP cenobamate 40 mg/kg on mean Racine seizure score (A), mean afterdischarge threshold (B), and mean afterdischarge duration (C) in (ADT study, n = 6/group). In the afterdischarge threshold test for hippocampal kindled seizures, rats were implanted with ventral hippocampal bipolar electrodes, allowed to recover, and then kindled to a Stage 5 Racine behavioral score. After another week of recovery, the individual afterdischarge threshold of each rat was determined by increasing the current intensity delivered via a ventral hippocampal bipolar electrode in a stepwise fashion until the rat displayed an electrographic afterdischarge with duration of at least 4 s. The initial stimulation was conducted at an intensity of 20 μA and increased in 10 μA increments every 1–2 min until an afterdischarge was elicited. Fifteen minutes after the pre-drug threshold determination, a single dose of 40 mg/kg cenobamate was administered intraperitoneally to a group of 6 rats. The individual rat afterdischarge threshold (B) was then redetermined at four timepoints (ie, 0.25, 1, 2, and 4 h) after drug administration. The Racine seizure score (A) and afterdischarge duration (C) were also recorded at the afterdischarge threshold. The results indicate that for time points from 15 min to 1 h post-dose, the increased threshold effect was statically significant and corresponding measurements for those same time periods showed significance for decreased duration of seizure activity and actual Racine seizure scores. Data reported as mean ± standard error of the mean. *Indicating statistically significant differences (P < 0.05) from Time 0.0.
3.4. GAERS model
After IP administration to rats, cenobamate at doses of 20 and/or 30 mg/kg showed significant dose-dependent reductions in the number (Fig. 3A) and cumulative duration (Fig. 3B) of SWDs relative to vehicle. The decrease in the number of SWDs between 30 and 50 min post-dose at 30 mg/kg of IP cenobamate was maintained through the rest of the 90-min post-dose observation period. The decrease in the cumulative durations of SWDs occurred from 30 to 70 min at 20 mg/kg cenobamate and from 10 to 90 min at 30 mg/kg cenobamate. The highest tested dose of cenobamate, 30 mg/kg IP, produced a near maximal reduction in the number (Fig. 3A) and cumulative duration of SWDs (Fig. 3B). The number of SWDs was significantly reduced in the valproate 150 mg/kg group at 30–50 min after injection compared to vehicle, while for 200 mg/kg, valproate reduced SWDs for more of the post-treatment period (30–90 min after injection; data not shown). In the valproate 150 mg/kg condition, cumulative duration of SWDs was significantly reduced at time points 30–70 min after injection of 150 mg/kg valproate, as compared to vehicle. For animals administered 200 mg/kg valproate, the cumulated duration of SWDs was significantly reduced as compared to the vehicle condition for the whole post-treatment period (10–90 min after injection). The maximal reduction for both parameters and doses of valproate was at 30–50 min post-injection but was not fully maintained through 90 min post-injection. Multiple Freidman's tests on the number of SWDs per 20-min period indicated significant differences between the treatments at each time point except baseline (Q-values: Baseline: Q = 12.46, p = 0.0525; 10–30 min: Q = 22.25, p = 0.0011; 30–50 min: Q = 39.92, p < 0.0001; 50–70 min: Q = 34.12, p < 0.0001; 70–90 min: Q = 36.17, p < 0.0001). Multiple Friedman's tests on the cumulated duration of SWDs per 20-min period indicated significant differences between the treatments at each timepoint except baseline (Q-values: Baseline: Q = 3.376, p = 0.7604; 10–30 min: Q = 33.47, p < 0.0001; 30–50 min: Q = 45.29, p < 0.0001; 50–70 min: Q = 38.75, p < 0.0001; 70–90 min: Q = 34.72, p < 0.0001).
Fig. 3.
Number of SWDs (A) and cumulative durations of SWDs (B) during baseline and post treatment periods in IP vehicle and cenobamate (n = 9/group; Study M). In the Genetic Absence Epilepsy Rat from Strasbourg (GAERS) Model, rats were implanted with EEG-recording electrodes and after a week recovery, animals were subjected to each treatment condition at least 71 h apart in a cross-over design. Rats were maintained in a quiet wakefulness state within an EEG recording chamber for an hour baseline period prior to IP administration and then for 90 min post IP administration. (A.) Number of spike-and-wave discharges (SWDs) (mean ± SEM, n = 9) during baseline (20 min pre-IP administration) and post-treatment periods in vehicle and cenobamate (5, 10, 20, and 30 mg/kg) conditions. At 30 mg/kg cenobamate, there was an increasingly significant reduction in the number of SWDs. (B.) Cumulative durations of SWDs (mean ± SEM, n = 9) during baseline and post-treatment periods in vehicle and cenobamate (5, 10, 20, and 30 mg/kg). At 20 and 30 mg/kg cenobamate, there was an increasingly significant reduction in the cumulative duration of SWDs. **Indicating statistically significant differences (P < 0.01) relative to vehicle. ***Indicating statistically significant differences (P < 0.001) relative to vehicle. ****Indicating statistically significant differences (P < 0.0001) relative to vehicle.
3.5. Rotarod test and minimal motor impairment
The median TD50 for cenobamate in rats ranged between 51 and 348 mg/kg PO (assessed at 1-, 2-, and 4-h post-dose), with a peak activity time of 1-h post-dose. In the study comparing three batches of cenobamate, the median TD50 values based on rotarod impairment for the three batches were considered similar (100.4, 104.1, and 101.6 mg/kg PO). When TD50 values were based on minimal motor impairment, cenobamate showed median TD50 values of 50.7 mg/kg PO and 38.9 mg/kg IP. YKP3090 had a median TD50 of <150 and 50.9 mg/kg when administered PO and IP, respectively. YKP1983 had a median TD50 of 81.1 mg/kg when administered PO and 53.3 mg/kg when administered IP (Table 9).
Table 9.
Effect of cenobamate, YKP3090, and YKP1983 on the rotarod test and minimal motor impairment in rats (multiple studies).
| Study Type (STUDY) | Test Compound | Route & Dose | N per dose tested | Time of Test (post-dose in h) | TD50 mg/kg (95% CI) |
|---|---|---|---|---|---|
| Rotarod test (Q) | Cenobamate | PO 150, 200, 250 mg/kg | 8 | 4 | 195.7 (158.0–242.5) |
| Rotarod test (R) | Cenobamate | PO 100, 150, 200, 250 mg/kg | 6 | 1 2 |
347.5 (146.7–823.2) 244.4 (88.2–677.2) |
| Rotarod test (S) | Cenobamate | PO 10, 30, 100, 250 mg/kg (3 batches 1401-1401-07-001; 1401-1401-05-501; DIT040503) | 6 | 0.5 to 5 | 101.6 (27.9–220.1) |
| 6 | 104.1 (40.0–204.4) | ||||
| 6 | 100.4 (−2.0-305.9) | ||||
| Minimal motor impairment (NINDS) | Cenobamate | PO 25, 50, 75, 100 mg/kg | 8 | 1 | 50.7 (35.7–63.0) |
| YKP3090 | PO 50, 100, 150 mg/kg | 8 | 1 | <150a (N/A) | |
| YKP1983 | PO 50, 75, 120 mg/kg | 8 | 1 | 81.1 (61.1–112) | |
| Cenobamate | IP 15, 30, 40, 50, 60 mg/kg | 8 | 0.25 | 38.9 (32.2–43.9) | |
| YKP3090 | IP 10, 20, 25, 50,75, 100 mg/kg | 8 | 0.5 | 50.9 (33.1–67.8) | |
| YKP1983 | IP 2, 7.5, 8, 15, 30, 50, 60, 73 mg/kg | 4–12 | 0.5 | 53.3 (41.5–62.2) |
aTD50 could not be calculated but estimated to be 150 mg/kg PO or less; h, hours; IP, intraperitoneal; NINDS, National Institute of Neurological Disorders and Stroke; PO, oral; RT, rotarod test; TD50, median neurotoxic dose.
4. Discussion
Cenobamate showed efficacy or signals of efficacy in all of the rodent seizure and epilepsy models evaluated at doses producing little to no behavioral impairment. In contrast, YKP3090 and YKP1983 were active only in generalized seizure and epilepsy models in mice and rats, but not in the rat hippocampal kindling model. While both enantiomers are active, cenobamate was considered more broadly active, which led to further clinical development and the subsequent New Drug Application and European Medicines Agency submissions. Furthermore, cenobamate had a favorable risk versus benefit ratio as the median neurotoxic dose was between 50 and 350 mg/kg, doses greater than those required to elicit anticonvulsant effects, and the PI in most of the studies was greater than one.
Results from the MES and IV PTZ seizure threshold tests suggest that cenobamate exerts its antiseizure effect by preventing seizure spread and increasing seizure threshold, respectively. In addition, cenobamate displayed dose-dependent inhibition of seizures in a number of other seizure tests including: the SC bicuculline, picrotoxin, and PTZ tests; 6 Hz psychomotor seizure test; and GAERS without potentiating seizure initiation (hippocampal kindling model) or the development of tolerance to its anticonvulsant effects (MES test). As cenobamate increased the seizure threshold for PTZ, it is not expected to have any notable negative effects on the seizure threshold. These data suggest that there would be a low risk of proconvulsant activity in humans; albeit, one should still consider caution when initiating treatment with cenobamate or any other ASM. Additionally, cenobamate inhibited 6 Hz limbic seizures at all three stimulus intensities tested without a shift in potency. The anticonvulsant effects in rodents occurred with cenobamate doses between 3 and 30 mg/kg, which approximate to 2.9 mg/kg and 5.7 mg/kg for a 200 mg and 400 mg dose in an adult human (based on a weight of 70 kg), respectively.
Overall, cenobamate was found to have activity in animal models of focal and generalized seizures, including absence seizures. The activity in focal seizure models is consistent with the results of two pivotal phase 2, double-blind, randomized, clinical studies (C013, Chung et al., 2020 [35]; C017, Krauss et al., 2020 [36]) of adjunctive cenobamate compared with placebo (both in combination with one to three ASMs) in patients with uncontrolled focal onset seizures. In both studies, there was a statistically significant reduction in seizure frequency (median percent reduction per 28 days versus baseline) during the double-blind period and higher rates of responders (≥50% reduction in seizure frequency from baseline) during the maintenance phase [[35], [36], [37]]. Furthermore, in both studies a significant percentage of patients experienced zero seizures (seizure freedom) during the maintenance phase (C013: 28.3% cenobamate 200 mg/day versus 8.8% placebo; C017: 11.2% cenobamate 200 mg/day and 21.1% cenobamate 400 mg/day versus 1.0% placebo) [35,36]. In a recent review and network meta-analysis [38], cenobamate ranked the highest in efficacy over 4 other third generation ASMs.
The motor coordination test results also aligned with data from the two phase 2 clinical studies and a phase 3 long-term open-label safety study (C021, [39]). The most common treatment-emergent adverse events in the clinical studies were central nervous system-related events such as somnolence, dizziness, and fatigue, which increased in incidence with increases in the cenobamate dose in the C017 study [35,36,39]. For example, the percent of patients with somnolence was 19%, 21%, and 37% for the 100, 200, and 400 mg/day cenobamate dose, respectively (8% in the placebo group) [36].
In summary, animal model data showed cenobamate to be a broad-spectrum ASM, with minimal behavioral impairment, supporting its use in the treatment of partial-onset (focal) seizures and warranting further research in generalized and absence seizures.
Data availability statement
Data will be made available on request.
Ethics statement
All studies were carried out in accordance with the National Research Council Publication “Guide for the Care and Use of Laboratory Animals” as adopted and promulgated by the NIH and were approved by the respective Institution's Animal Care and use Committee or local equivalent.
Author contributions
Susan M. Melnick, Yujin Shin: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Kelli J. Glenn: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Role of the funding source
This work was supported by SK Biopharmaceuticals, Co., Ltd. (New Jersey, US [now known as SK Life Science, Inc.] and Seongnam, Gyeonggi, Republic of Korea) and in part by the Epilepsy Therapy Screening Program (through the National Institute of Neurological Disorders and Stroke (NINDS) of the National Institutes of Health (NIH) in the US [Contract #NO1-NS-4-2359]. The study funder either designed, conducted, analyzed the data and documented study results or consulted with advisors and/or study investigators on the design of the original study and post hoc analysis, provided financial and/or material support for the study, and, with the assistance of study investigators, monitored the conduct of the study, collected data from the investigative centers, and analyzed the data. SMM, YS, and KJG are employees of the sponsor and as authors had a role in data analysis, data interpretation, and writing of the report. The authors had full access to all study data and had final responsibility for the decision to submit for publication. All authors approved the final version of the manuscript for publication.
Previous presentation
The GAERS model data was submitted in part as an abstract and accepted and presented as a poster at the Society for Neuroscience (SfN) 2019 Annual Meeting, October 19–23, Chicago, IL, US.
Data sharing
The data for the analyses described in this manuscript are available by request from the corresponding author or SK Life Science, Inc., the company sponsoring the clinical development of cenobamate for the treatment of partial-onset (focal) epilepsy.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Susan M. Melnick reports financial support was provided by SK Life Science, Inc. Yujin Shin reports financial support was provided by SK Biopharmaceuticals, Co., Ltd. Kelli J. Glenn reports financial support was provided by SK Life Science, Inc.
Acknowledgments
Cenobamate, YKP3090, and YKP1983 were synthesized at or for SK Life Science, Inc. (US)/SK Biopharmaceuticals (Korea). These studies were supported by SK Life Science, Inc. (Paramus, NJ, US) and/or by SK Biopharmaceuticals, Co., Ltd. (Seongnam, Gyeonggi, Republic of Korea). The GAERS study was designed and conducted by SynapCell SAS (Saint Ismier, France). Medical writing assistance provided by Dorothy McCoy, PharmD, BCPS, BCIDP and professional editorial and submission assistance by Courtney Breuel of MedVal Scientific Information Services, LLC (Princeton, NJ, US) and was funded by SK Life Science, Inc. SK Life Science, Inc. gratefully acknowledges the contributions of Dr. S. James Lee, Dr. Robert Gordon, Dr. Hyewon Shin, Dr. Gregory Ervin, Ray Nemzek, and the Epilepsy Therapy Screening Program at the National Institute of Health/National Institute of Neurological Disorders and Stroke. SK Life Science, Inc. greatly appreciates the efforts of Dr. H. Steve White who directed the Epilepsy Therapy Screening Program during the development of cenobamate and reviewed the current manuscript.
References
- 1.Perucca E., Brodie M.J., Kwan P., Tomson T. 30 years of second-generation antiseizure medications: impact and future perspectives. Lancet Neurol. 2020;19(6):544–556. doi: 10.1016/S1474-4422(20)30035-1. [DOI] [PubMed] [Google Scholar]
- 2.Perucca P., Scheffer I.E., Kiley M. The management of epilepsy in children and adults. Med. J. Aust. 2018;208(5):226–233. doi: 10.5694/mja17.00951. [DOI] [PubMed] [Google Scholar]
- 3.Golyala A., Kwan P. Drug development for refractory epilepsy: the past 25 years and beyond. Seizure. 2017;44:147–156. doi: 10.1016/j.seizure.2016.11.022. [DOI] [PubMed] [Google Scholar]
- 4.Perucca P., Gilliam F.G. Adverse effects of antiepileptic drugs. Lancet Neurol. 2012;11(9):792–802. doi: 10.1016/S1474-4422(12)70153-9. [DOI] [PubMed] [Google Scholar]
- 5.Glauser T., Ben-Menachem E., Bourgeois B., et al. ILAE treatment guidelines: evidence-based analysis of antiepileptic drug efficacy and effectiveness as initial monotherapy for epileptic seizures and syndromes. Epilepsia. 2006;47(7):1094–1120. doi: 10.1111/j.1528-1167.2006.00585.x. [DOI] [PubMed] [Google Scholar]
- 6.Hauser W.A. Questioning the effectiveness of newer antiseizure medications. JAMA Neurol. 2018;75(3):273–274. doi: 10.1001/jamaneurol.2017.3069. [DOI] [PubMed] [Google Scholar]
- 7.Loscher W. Animal models of seizures and epilepsy: past, present, and future role for the discovery of antiseizure drugs. Neurochem. Res. 2017;42(7):1873–1888. doi: 10.1007/s11064-017-2222-z. [DOI] [PubMed] [Google Scholar]
- 8.Bialer M., Johannessen S.I., Levy R.H., Perucca E., Tomson T., White H.S. Progress report on new antiepileptic drugs: a summary of the Eleventh Eilat Conference (EILAT XI) Epilepsy Res. 2013;103(1):2–30. doi: 10.1016/j.eplepsyres.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 9.Bialer M., Johannessen S.I., Levy R.H., Perucca E., Tomson T., White H.S. Progress report on new antiepileptic drugs: a summary of the Ninth Eilat Conference (EILAT IX) Epilepsy Res. 2009;83(1):1–43. doi: 10.1016/j.eplepsyres.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 10.Nakamura M., Cho J.H., Shin H., Jang I.S. Effects of cenobamate (YKP3089), a newly developed anti-epileptic drug, on voltage-gated sodium channels in rat hippocampal CA3 neurons. Eur. J. Pharmacol. 2019;855:175–182. doi: 10.1016/j.ejphar.2019.05.007. [DOI] [PubMed] [Google Scholar]
- 11.Bialer M., Johannessen S.I., Koepp M.J., et al. Progress report on new antiepileptic drugs: a summary of the Fifteenth Eilat Conference on New Antiepileptic Drugs and Devices (EILAT XV). II. Drugs in more advanced clinical development. Epilepsia. 2020;61(11):2365–2385. doi: 10.1111/epi.16726. [DOI] [PubMed] [Google Scholar]
- 12.Guignet M., Campbell A., White H.S. Cenobamate (XCOPRI): can preclinical and clinical evidence provide insight into its mechanism of action? Epilepsia. 2020;61(11):2329–2339. doi: 10.1111/epi.16718. [DOI] [PubMed] [Google Scholar]
- 13.Sharma R., Nakamura M., Neupane C., et al. Positive allosteric modulation of GABAA receptors by a novel antiepileptic drug cenobamate. Eur. J. Pharmacol. 2020;879 doi: 10.1016/j.ejphar.2020.173117. [DOI] [PubMed] [Google Scholar]
- 14.Klitgaard H., Matagne A., Nicolas J.M., et al. Brivaracetam: rationale for discovery and preclinical profile of a selective sv2a ligand for epilepsy treatment. Epilepsia. 2016;57(4):538–548. doi: 10.1111/epi.13340. [DOI] [PubMed] [Google Scholar]
- 15.White H.S. Clinical significance of animal seizure models and mechanism of action studies of potential antiepileptic drugs. Epilepsia. 1997;38(suppl 1):S9–S17. doi: 10.1111/j.1528-1157.1997.tb04523.x. [DOI] [PubMed] [Google Scholar]
- 16.Scheffer I.E., Berkovic S., Capovilla G., et al. ILAE classification of the epilepsies: position paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58(4):512–521. doi: 10.1111/epi.13709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Woodbury L.A., Davenport V.D. Design and use of a new electroshock seizure apparatus, and analysis of factors altering seizure threshold and pattern. Arch. Int. Pharmacodyn. Ther. 1952;92(1):97–107. [PubMed] [Google Scholar]
- 18.Kupferberg H.J. Antiepileptic drug development program: a cooperative effort of government and industry. Epilepsia. 1989;30(suppl 1):S51–S56. doi: 10.1111/j.1528-1157.1989.tb05815.x. ; discussion S64-S68. [DOI] [PubMed] [Google Scholar]
- 19.Swinyard E.A., Sofia R.D., Kupferberg H.J. Comparative anticonvulsant activity and neurotoxicity of felbamate and four prototype antiepileptic drugs in mice and rats. Epilepsia. 1986;27(1):27–34. doi: 10.1111/j.1528-1157.1986.tb03497.x. [DOI] [PubMed] [Google Scholar]
- 20.Sarma P., Anusuya B. Models of epilepsy used in antiepileptic drug discovery: a review. Int. J. Pharm. Pharm. Sci. 2014;6(11) [Google Scholar]
- 21.White H.S., Wolf H.H., Woodhead J.H., Kupferberg H.J. The National Institutes of Health anticonvulsant drug development program: screening for efficacy. Adv. Neurol. 1998;76:29–39. [PubMed] [Google Scholar]
- 22.Toman J.E., Everett G.M., Richards R.K. The search for new drugs against epilepsy. Tex. Rep. Biol. Med. 1952;10(1):96–104. [PubMed] [Google Scholar]
- 23.Barton M.E., Klein B.D., Wolf H.H., White H.S. Pharmacological characterization of the 6 Hz psychomotor seizure model of partial epilepsy. Epilepsy Res. 2001;47(3):217–227. doi: 10.1016/s0920-1211(01)00302-3. [DOI] [PubMed] [Google Scholar]
- 24.Lothman E.W., Williamson J.M. Closely spaced recurrent hippocampal seizures elicit two types of heightened epileptogenesis: a rapidly developing, transient kindling and a slowly developing, enduring kindling. Brain Res. 1994;649(1–2):71–84. doi: 10.1016/0006-8993(94)91050-2. [DOI] [PubMed] [Google Scholar]
- 25.Lothman E.W., Salerno R.A., Perlin J.B., Kaiser D.L. Screening and characterization of antiepileptic drugs with rapidly recurring hippocampal seizures in rats. Epilepsy Res. 1988;2(6):367–379. doi: 10.1016/0920-1211(88)90048-4. [DOI] [PubMed] [Google Scholar]
- 26.Racine R.J. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr. Clin. Neurophysiol. 1972;32(3):281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- 27.Löscher W., Hönack D., Fassbender C.P., Nolting B. The role of technical, biological and pharmacological factors in the laboratory evaluation of anticonvulsant drugs. III. Pentylenetetrazole seizure models. Epilepsy Res. 1991;8(3):171–189. doi: 10.1016/0920-1211(91)90062-k. [DOI] [PubMed] [Google Scholar]
- 28.White H.S. Comparative anticonvulsant and mechanistic profile of the established and newer antiepileptic drugs. Epilepsia. 1999;40(suppl 5):S2–S10. doi: 10.1111/j.1528-1157.1999.tb00913.x. [DOI] [PubMed] [Google Scholar]
- 29.Vogel H.G. In: Drug Discovery and Evaluation: Pharmacological Assays. second ed. Vogel H.G., editor. Springer-Verlag Berlin Heidelberg; Germany: 2002. Picrotoxine induced convulsions; p. 424. [Google Scholar]
- 30.Orloff M.J., Williams H.L., Pfeiffer C.C. Timed intravenous infusion of metrazol and strychnine for testing anticonvulsant drugs. Proc. Soc. Exp. Biol. Med. 1949;70(2):254–257. doi: 10.3181/00379727-70-16891. [DOI] [PubMed] [Google Scholar]
- 31.Dunham N.W., Miya T.S. A note on a simple apparatus for detecting neurological deficit in rats and mice. J. Am. Pharm. Assoc. Am. Pharm. Assoc. 1957;46(3):208–209. doi: 10.1002/jps.3030460322. [DOI] [PubMed] [Google Scholar]
- 32.Litchfield J.T., Jr., Wilcoxon F. A simplified method of evaluating dose-effect experiments. J. Pharmacol. Exp. Ther. 1949;96(2):99–113. [PubMed] [Google Scholar]
- 33.Finney D.J. third ed. Cambridge University Press; Cambridge, UK: 1971. Probit Analysis. [Google Scholar]
- 34.Swinyard E.A., Woodhead J.H., White H.S., Franklin M.R. In: Antiepileptic Drugs. third ed. Levy R., Mattson R., Meldrum B., Penry J.K., Dreifuss F.E., editors. Raven Press, Ltd.; New York: 1989. General principles: experimental selection, quantification, and evaluation of anticonvulsants; pp. 85–102. [Google Scholar]
- 35.Chung S.S., French J.A., Kowalski J., et al. Randomized phase 2 study of adjunctive cenobamate in patients with uncontrolled focal seizures. Neurology. 2020;94(22):e2311–e2322. doi: 10.1212/WNL.0000000000009530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krauss G.L., Klein P., Brandt C., et al. Safety and efficacy of adjunctive cenobamate (YKP3089) in patients with uncontrolled focal seizures: a multicentre, double-blind, randomised, placebo-controlled, dose-response trial. Lancet Neurol. 2020;19(1):38–48. doi: 10.1016/S1474-4422(19)30399-0. [DOI] [PubMed] [Google Scholar]
- 37.Klein P., Ferrari L., Rosenfeld W.E. Cenobamate for the treatment of focal seizures. US Neurol. 2020;16(2):87–97. [Google Scholar]
- 38.Lattanzi S., Trinka E., Zaccara G., et al. Third-generation antiseizure medications for adjunctive treatment of focal-onset seizures in adults: a systematic review and network meta-analysis. Drugs. 2022;82(2):199–218. doi: 10.1007/s40265-021-01661-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sperling M.R., Klein P., Aboumatar S., et al. Cenobamate (YKP3089) as adjunctive treatment for uncontrolled focal seizures in a large, phase 3, multicenter, open-label safety study. Epilepsia. 2020;61(6):1099–1108. doi: 10.1111/epi.16525. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.