Abstract
Herein, copper indium diselenide ternary (CuInSe2) thin film has been deposited on Indium Tin Oxide (ITO) coated glass substrate by electrochemical deposition technique with different potential and pH solutions. CuInSe2 thin films were deposited by one-step electrodeposition before post-depot selenization at 450 °C for 30 min. The effect of potential and pH on the structural and optical properties of CuInSe thin film have been studied using X-ray diffraction (XRD), Scanning electron microscopy (SEM), and UV–Visible spectrometer. According to the X-ray diffraction (XRD) measurements, it was observed that all samples exhibit prominent reflections (112), (204/220), and (312/116) of tetragonal CuInSe2. The films electrodeposited at −0.8 V potential shows growth and peak values increasing in the (204/220) crystal direction within a pH range of 2.2, whereas the films electrodeposited at pH 2.6 tend to favor an increase in (112) peaks. We also noticed an improvement in surface morphology and adherent of CuInSe2 thin films electrodeposited at −0.8 V applied potential from the solution having pH 2.6. The band gaps of samples electrodeposited at −0.8V potentials from pH 2.6, 2.4, and 2.2 solutions were 1.15 eV, 1.25 eV, and 1.21 eV, respectively. As part of our investigation, we used a Solar Cell capacitance simulator (SCAPS) to perform our electrodeposited films. The most effective Power conversion efficiency (PCE) was obtained for thin films electrodeposited at −0.8 V within the solution having pH 2.4.
Keywords: Potential, pH, Copper indium diselenium ternary (CISe), Electrodeposition
1. Introduction
CIGS-based thin-film solar cells are continuously being explored to improve thin-film solar cell efficiency. New records in terms of efficiency and productivity are achieved almost every year. The Centre for Solar Energy and Hydrogen Research Baden-Württemberg (ZSW) has improved its thin film CIGS record power conversion efficiency (PCE) of 0.9% from the previous value of 20.8% in 2014 [1]. The latest Solibro published record is 18.72% CIGS module efficiency on a 0.94 m2 [2]. In 2019, Solar Frontier K.K. achieved a record power conversion efficiency of 23.35% on a 1 cm2, exceeding the 22.9% achieved in 2017 [3]. These reported values remain quite close to the result obtained from the crystalline silicon solar cell, which is about 26.7% for crystalline silicon cells [4]. In these solar cells, CIGS absorbers can absorb over 90% of solar radiation with a thickness of only 2–3 μm and even with a thickness of 1 μm [5]. There are different phases of this alloy depending on the growth conditions. The substrate, element flux, and temperature are some of the parameters that affect the phases and properties of the alloy during growth. The high efficiency of these materials was generally produced using vacuum techniques. However, CuInSe and thin films are classified as photovoltaic materials with a low-cost elaboration because of the large variety of deposition techniques with high-efficiency including non-vacuum technique such as spray pyrolysis, spin coating, and electrodeposition [[5], [6], [7], [8], [9], [10]]. Electrodeposition is a suitable process for growing an economically large area of CIGS thin film. Electrodeposited Cu–In–Se layers have achieved efficiencies up to 14% for a small area and efficiencies exceeding 11% for a large area [11]. The Deposition of CIS and CIGS absorber layer can be electrodeposited in one set or by two sets [12,13]. However, several parameters such as electrolyte composition, deposition pH and the potential need to be controlled to obtain adequate thickness and good structural and optical properties of deposited thin films. Changing these parameters leads to a significant variation in the material properties. In the case of one set process, these parameters need to be adjusted using complexing agents so that all metal precursors' reduction potential becomes closer [14]. The two-step process involves the deposition of pure elements or combinations with binary or ternary stacked films structure, followed by the selenization or sulfurization of the films [12,15].
The majority of studies have examined the co-deposition (one-step) potential of elements, but very few have examined subsequently the impact of both pH and applied potential on the crystal orientation, optical band gap, and solar cell performance on CuInSe2 thin films electrodeposited [[16], [17], [18]]. In this work, we investigate the effects the effect of potential and pH on the structural and optical properties of CuInSe2 (CISe) absorber layer after co-deposition (one-step) Cu–In–Se layers and selenization. We also exhibit optical bandgap parameters and performed the solar cell by using SCAPS 1-D.
2. Experimental method
CuInSe2 (CISe) films are grown on tin oxide (ITO) coated glass substrate. The electrochemical deposition technique has been used by applying a different value of potential and pH. The electrochemical setup consists of a tin oxide-coated glass substrate (ITO), an Ag/AgCl electrode, and a platinum (Pt) electrode used as working, reference, and counter-electrodes respectively. The substrates were pre-treated with 2 ml of Hellmanex III dissolved in distilled water and sonicated for 15 min in acetone, ethanol, and isopropanol. Subsequently, the substrates were dried and finally subjected to UV light in a UV ozone cleaner for 15 min. Different electrolytic baths containing 2.10−3 M copper chloride (CuCl2), 10.10−3 M indium tri-chloride (InCl3), 4.10−3 M selenous acid (H2SeO3) in an aqueous solution were prepared. Potassium thiocyanate KSCN (0.04 M), lithium chloride LiCl (0.3 M), and ammonium chloride NH4Cl2 (0.1 M) mixed electrolytes were used as a complexing agent, supporting electrolyte and brightener respectively. Potassium hydroxide (KOH) was used to adjust the pH of the bath at different deposition potentials (−0.7 V, −0.8 V, and −0.9 V). The electrodeposition process was performed for 60 min at room temperature. The optimization of the co-deposition process leads us to maintain the potential at −0.8V for different pH values 2.2 (set A), 2.4 (set B), and 2.6 (set C). The as-deposited films were annealed for 30 min at 450 °C in a tubular furnace under a selenium atmosphere and subsequently used for further characterization. Electrodepositing CISe involves the exchange of 13 electrons based on the following reaction (1):
| Cu2+ + In3+ + 2SeO32− + 12H+ + 13e− → CuInSe2 + 6H2O | (1) |
3. Results and discussion
3.1. Cyclic voltammetry
Fig. 1 shows the cyclic voltammetry (CV) of CuInSe2 aqueous solution in ambient temperature. The cathodic peak at −0.78 V is attributed to the reduction of the precursor bath as shown in equation (1) with a pH of around 2.4, demonstrating that CuInSe2 can be successfully deposited on an ITO-based glass substrate. In order to analyze the impact of both pH and applied potential various batch of solutions has been prepared, and Potassium hydroxide (KOH) was used to adjust the pH values (2.2, 2.4, and 2.6) at different applied potentials (−0.7 V, −0.8 V, and −0.9 V).
Fig. 1.
CuInSe2 CV in combined precursor baths.
3.2. Structural measurements
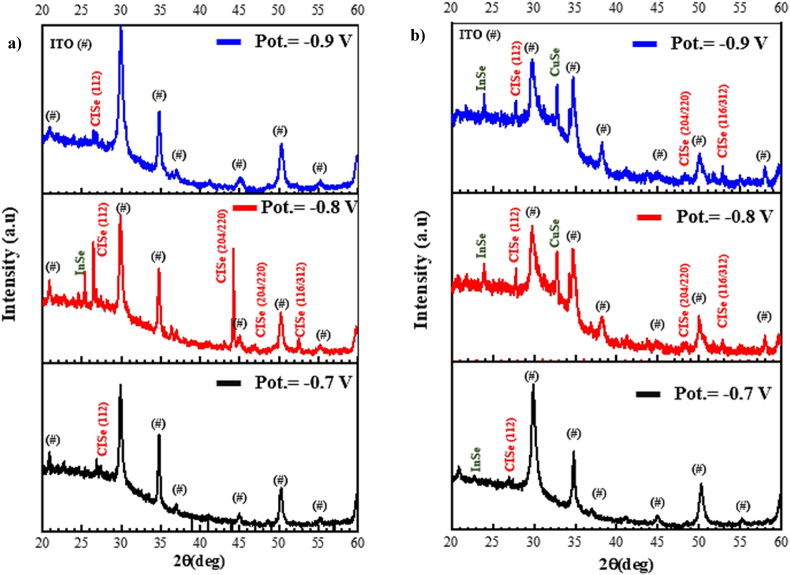
The crystallinity with different applied potential and pH values can be seen in the XRD results shown in Fig. 2, Fig. 3. The peaks identified with (#) indicate peaks from ITO substrate. It is seen that the preferential orientation (112) of CuInSe2 can be seen in all samples. At −0.7V, similar peak intensity is observed for the CISe (112) peak at pH 2.4, as seen at pH 2.2, where the In2Se3 (InSe) phase can also be observed. At −0.8V, peak intensities are more significant with a growing film in the CISe (112) direction at pH 2.4. Furthermore, the planes (204/220), and (116/312) of CISe appear in all electrodeposited films at −0.8 V deposition potential. At −0.9V, similar behavior can be observed at −0.8 v and −0.7 v for pH values 2.2 and 2.4 respectively.
Fig. 2.
XRD patterns of electrodeposited CISe thin films with pH (a) 2.4 and (b) 2.2 at −0.7 V, −0.8 V, and −0.9V applied potential.
Fig. 3.
XRD patterns of CuInSe2 thin films electrodeposited at −0.8 V applied potential from 2.2, 2.4, and 2.6 pH solution.
We found that as well as the pH of the bath remaining constant, the peaks vary with the deposition potential, and increasing applied potential leads to poor adherence of as-deposited films. We observed that the crystallinity tends to improve for −0.8V, and −0.9V with binary InSe and Cu3Se2 (CuSe) phases (JCPDS No. 85–0184, JCPDS No. 86–1240), and more preferential orientation peaks for −0.8V [19].
Based on this analysis, the deposition potential has been fixed to −0.8V (vs. SCE). In order to analyze the impact of pH on deposition setup and electrodeposited film properties different solutions of 2.2, 2.4, and 2.6 pH values were prepared. It is observed from Fig. 3 that the intensity of some of the peaks changes with pH. Cu, In, and Se co-deposition potentials at −0.8V lead to film growing in the preferential direction (112) when the pH value changes from 2.2 to 2.6 as reported in Refs. [[20], [21], [22]]. XRD results indicate that typical peaks of the chalcopyrite structure, (112), (204/220), and (312/116) start showing up in the sample deposited at a pH value of 2.4. As the pH of the bath changes, the intensity of these peaks increases, with a strong peak in the (112) preferential orientation without any secondary phases at 2.6 pH. CuInSe2 films electrodeposited at pH 2.4 show growth and increased crystallinity in (204/220) crystal direction whereas the films electrodeposited at pH 2.6 depict the direction along (112).
3.3. FWHM and crystal size
The crystallinity parameters of CuInSe2 thin films electrodeposited at potentials −0.8 V from the solution having pH 2.2 (set A), 2.4 (set B), and 2.6 (set C) were obtained from XRD results, as shown in Table 1.
Table 1.
Crystal parameters of CuInSe2 thin films electrodeposited at potentials −0.8 V from the solution having pH 2.2 (set A), 2.4 (set B), and 2.6 (set C).
| Samples ID | 2 theta | hkl | β(FWHM) | d (Ang) | D (nm) | δ × 10−3 (nm−2) | ε × 10−3 |
|---|---|---|---|---|---|---|---|
| Set A | 26.76 | 112 | 0.22 | 3.33 | 39.40 | 0.64 | 23.13 |
| 44.73 | 204/220 | 0.94 | 2.02 | 9.50 | 11.08 | 57.14 | |
| Set B | 26.79 | 112 | 0.12 | 3.32 | 70.70 | 0.20 | 12.60 |
| 44.19 | 204/220 | 0.06 | 2.04 | 138.00 | 0.05 | 3.70 | |
| 52.53 | 116/312 | 0.16 | 1.74 | 57.00 | 0.31 | 8.11 | |
| Set C | 26.34 | 112 | 0.24 | 3.38 | 35.30 | 0.80 | 25.65 |
| 44.05 | 204/220 | 0.35 | 2.05 | 25.60 | 1.52 | 21.64 | |
| 52.39 | 116/312 | 0.44 | 1.74 | 21.10 | 2.24 | 22.37 |
Based on the grain size, we can determine the dislocation density per unit volume of the crystal using equations (2), (3) from Williamson and Smallman [20,23]:
| (2) |
The grain deformation ε of the films is examined according to the β values obtained for each angle according to equation (3) [24]:
| (3) |
3.4. Morphology and distribution of elements
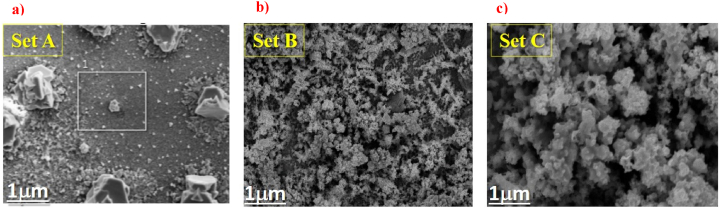
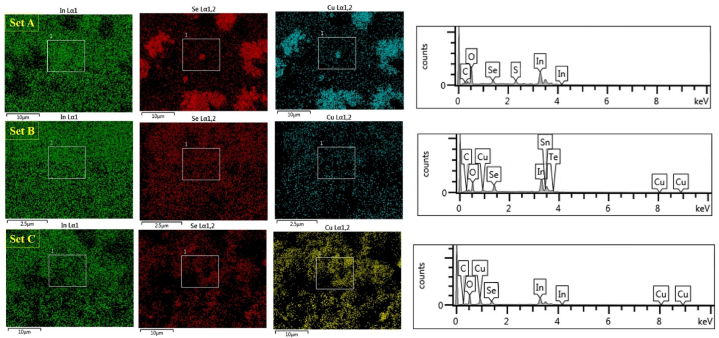
Fig. 4, Fig. 5 show the SEM images and X-ray Energy Dispersive Spectroscopy (EDS) elemental mapping of CuInSe2 thin films electrodeposited at potentials −0.8 V from the solution having pH 2.2 (set A), 2.4 (set B), and 2.6 (set C) with the presence of others elements (O, C, S …). The improvement in surface morphology and the growth process is evident in Fig. 5 when the pH increases from 2.2 to 2.6.
Fig. 4.
SEM image of Cu–In–Se layers electrodeposited at potentials −0.8 V from the solution having pH a) 2.2 (set A), b) 2.4 (set B), and c) 2.6 (set C).
Fig. 5.
SEM image and EDS elemental mapping of Cu–In–Se layers electrodeposited at potentials −0.8 V from the solution having pH 2.2 (set A), 2.4 (set B), and 2.6 (set C).
Table 2 shows the elemental concentrations of each Cu, In, and Se elements for CuInSe2 thin films electrodeposited at −0.8 V applied potentials with 2.2, 2.4, and 2.6 pH values.
Table 2.
Chemical composition of CuInSe2 thin films electrodeposited at potentials −0.8 V from the solution having pH 2.2 (set A), 2.4 (set B), and 2.6 (set C) from Energy-dispersive X-ray Spectroscopy (EDS).
| pH of the bath | Potential applied (V) | Atomic percentages (%) |
||
|---|---|---|---|---|
| Cu | In | Se | ||
| 2.2 (set A) | −0.8 | 3.12 | 85.26 | 11.62 |
| 2.4 (set B) | 9.31 | 71.86 | 18.83 | |
| 2.6 (set C) | 12.89 | 68.42 | 18.74 | |
The electrodeposition thin films at 2.2, and 2.4 pH values are highly rich in indium as determined by EDS, in agreement with XRD which can be associated to the electrolytic bath composition. However, as pH increases Cu and Se concentrations become more important so that the stoichiometry of the CISe layer is improved. This could be associated to the improvement of complexing agents’ ability to form complexes and the adjustment of the reduction potential of precursors [22].
3.5. Optical proprieties CuInSe2
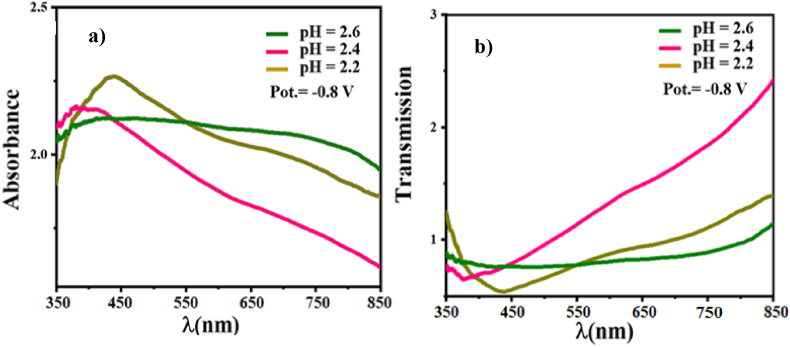
UV–vis spectrophotometer was used to record CuInSe2 thin films’ electrodeposited absorption and transmittance spectrum in the range of 350–850 nm for a solution having pH 2.2 (set A), 2.4 (set B), and 2.6 (set C) at potentials −0.8 V. Fig. 6a and 6b shows that all the materials have good absorption and transmission in the visible region as an absorber layer.
Fig. 6.
a) absorbance and b) transmission spectra of CuInSe2 thin films electrodeposited at potentials −0.8 V from the solution having pH 2.2 (set A), 2.4 (set B), and 2.6 (set C).
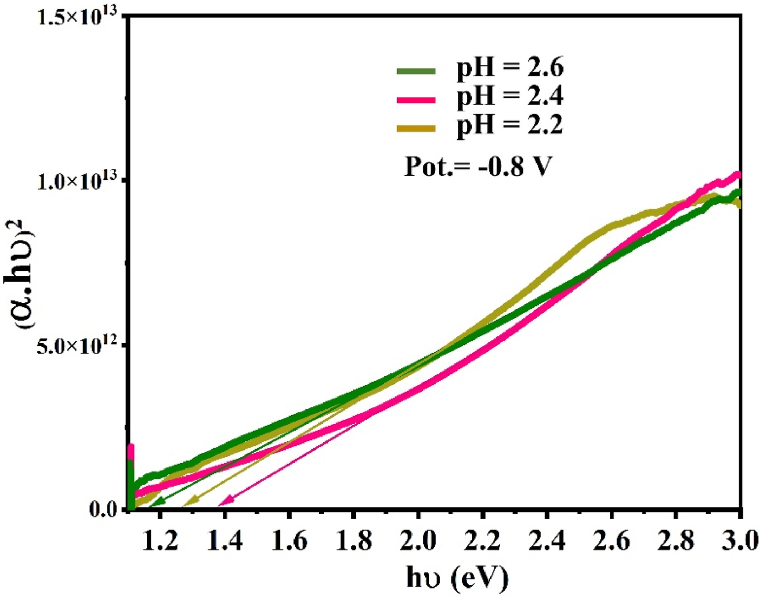
The bandgap values, as shown in Fig. 7, were obtained from absorption spectra data converted into vs plot using the Tauc equation (4) which matches well with other methods used in Refs. [23,24].
| (4) |
h, A, and ν refer to Planck's constant, a constant, and photon frequency, respectively; n is a constant related to electron transitions. The band gap values were found to be 1.16 eV, 1.38 eV, and 1.26 eV for samples electrodeposited at potentials −0.8 V from the solution having pH 2.6 (set C), 2.4 (set B), and 2.2 (set A), respectively. A similar optical properties behavior of electrodeposited films at different pH values was reported in Refs. [21,22,25,26]. As we can observe, the change in bandgap can be attributed to structural modifications arising as a result of grain growth orientation of CISe films, moreover to secondary phases such as InSe and CuSe [[27], [28]]. This can explain why films deposited at 2.6 to secondary phases show the closest bandgap to values reported in the literature [22,29,30]. However, the absorption and transmission can be affected an increasing way when the pH decreases from 2.6 to 2.2 [21].
Fig. 7.
Optical bandgap vs of CuInSe2 thin films electrodeposited at potentials −0.8 V from the solution having pH 2.2 (set A), 2.4 (set B), and 2.6 (set C).
4. Numerical devices performance
4.1. Digital structure
CuInSe2 thin films electrodeposited at different pH values impact both the structural and optical properties. A numerical evaluation of CuInSe2 layers electrodeposited using SCAPS-1D was made in order to further analyze the impact of varying band gaps related to the pH solution. We used the same structure and parameters as SCAPS-1D CIGS (example CIGS.def) solar cells, which consists of the following stacking: back contact/p-CuInSe2/OVC/CdS/ZnO-i/Ag, as shown in Fig. 8. The front of the cell is illuminated under a spectrum of AM1.5G equivalent to a density of 100 mW/cm2 at 300 K. A list of the SCAPS-1D software simulation parameters included the Table 3 of varying band gap related to the different pH values solution at −0.8 V applied potential.
Fig. 8.
Schematic of back contact/CuInSe2/OVC/CdS/i-ZnO/Ag Solar Cell.
Table 3.
Band gap variation of CuInSe2 thin films electrodeposited at potentials −0.8 V from the solution having pH 2.2 (set A), 2.4 (set B), and 2.6 (set C).
| pH of the bath | Potential applied (V) | Bandgap (eV) |
|---|---|---|
| CuInSe2 | ||
| 2.2 (set A) | −0.8 | 1.26 |
| 2.4 (set B) | 1.38 | |
| 2.6 (set C) | 1.16 |
The solar cell studied in this simulation has front and back contact with four other layers where FTO coated glass substrate is the back contact et Ag the front one modeled in flat bande mode. i-ZnO is a transparent conductive oxide (TCO) with optical transparency of about 90% [31] and worked as a windows layer. In CIGS applications, CdS serves as a buffer layer that transports most incident photons to the CIGS absorber layer [32]. OVC (Ordered Vacancy Compound) OVC layer with a thickness of 15 nm and a higher gap energy than CuInSe2 [33] would reduce interfacial recombination [34,35]. SCAPS solves Poisson equations and continuity equations for one-dimensional electrons and holes based on equations (5), (6), (7) respectively [36,37]:
| (5) |
| (6) |
| (7) |
are the relative dielectric and vacuum constants, respectively, V the electric potential, p, and n are the carrier densities, NA and ND concentrations of acceptor and donor atoms, Jn and Jp are current densities of electrons and holes, G and Rp,n are the generations and recombination rates of holes and electrons. The current density for each type of carrier is given by one-dimensional transport equation (8) and (9):
| (8) |
| (9) |
4.2. Effect of pH variation on the performances of the devices
Fig. 9a and b shows J-V and P–V curves for CuInSe2, respectively, and illustrate how varying the pH of CuInSe2 electrodeposited at a constant potential affects the cell's performance. The most efficient current density (Jsc) and power density (P) are obtained when CuInSe2 is deposited at pH 2.4, resulting in the largest band gap (1.38 eV).
Fig. 9.
a) J-V and P–V curves of CuInSe2 thin films electrodeposited at potentials −0.8 V from the solution having pH 2.2 (set A), 2.4 (set B), and 2.6 (set C).
The simulation results, as illustrated in Fig. 9 and Table 4, show that the current density (Jsc) decreases as the open circuit voltage (Voc), fill factor (FF), and efficiency (Eta) increase so that when the pH value changes from 2.2 to 2.6 cells’ performance was affected by the bandgap. This is indicated by the efficiency of 10.45%, 11.50%, and 12.43% with 1.16 eV, 1.26 eV, and 1.38 eV bandgap at 2.6, 2.2, and 2.4 pH values respectively. Voc and FF are affected in an increasing way by the bandgap whereas Jsc is probably reduced by carrier recombination in the device, particularly at the CISe/OVC/CdS interfaces. Power density or power conversion efficiency increases as long as the bandgap value is closer to the optimal theoretical value (around 1.4 eV) [[38], [39], [40], [41], [42], [43], [44]].
Table 4.
Experimental Characteristics Parameters of CuInSe2 thin films electrodeposited at −0.8 V applied potential from the solution having pH 2.2 (set A), 2.4 (set B), and 2.6 (set C).
| Solar cell model | pH of the bath | Voc |
Jsc |
FF |
Eta |
|---|---|---|---|---|---|
| (V) | (mA/cm2) | (%) | (%) | ||
| ITO/CuInSe2/OVC/CdS/i-ZnO | 2.2 (set A) | 0.61 | 29.91 | 62.50 | 11.50 |
| 2.4 (set B) | 0.73 | 26.66 | 64.09 | 12.43 | |
| 2.6 (set C) | 0.52 | 33.17 | 60.44 | 10.45 |
In summary, a pH of around 2.4 is more suitable under the above experimental conditions. This is taking into account the deposition of the CuInSe2 layer and the solar cell's performance [[45], [46], [47]].
5. Conclusion
CuInSe2 thin films were successfully deposited by one-step electrodeposition onto ITO back contact at −07 V, −08V, and −0.9V potential. An aqueous bath with 2.2, 2.4, and 2.6 pH values were used at each applied potential as well and subsequently, the films were selenized at 450 °C for 30 min. According to the X-ray diffraction (XRD) measurements, it was found that CuInSe2 films electrodeposited at −0.8 V potential show growth and increased crystallinity in (204/220) crystal direction from the solution having pH 2.4, whereas the films electrodeposited at pH 2.6, tends to favor the direction along (112) with increasing pH values. Electrodeposited samples from solutions with 2.6, 2.4, and 2.2 pH values showed band gaps of 1.16 eV, 1.38 eV, and 1.26 eV, respectively. The 2.4 pH is more suitable under the above conditions considering the deposition of the CuInSe2 layer and solar cell's performance. The pH of the bath has an impact on the properties of the CuInSe2 electrodeposited film and thus on cell performance.
Author contribution statement
Armel Ignace N'guessan: conceived and designed the experiments; performed the experiments; analyzed and interpreted the data; wrote the paper, Amal Bouich: performed the experiments; Analyzed and interpreted the data; wrote the paper, supervision, Donalfologo Soro: analyzed and interpreted the data, Bernabé Mari Soucase: contributed reagents, materials, analysis tools, or data.
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
Author Armel Ignace N'Guessan acknowledges his Erasmus+ KA 107, The author Amal Bouich acknowledges the Ministerio de Ciencia e Innovación (Spain) (MCIN) for funding support through Margarita Salas Fellowship (MCIN/AEI/10.13039/501100011033). This work has been funded by the Ministerio de Ciencia e Innovación (Spain) and by the Spanish Agencia Estatal de Investigación through projects BESTMAT PID2019-107137RB-C21/AEI/10.13039/501100011033 and PID2019-107137RB-C22/AEI/10.13039/501100011033 and by ERDF under the funding “A way of making Europe”.
References
- 1.Jackson P., Hariskos D., Wuerz R., Kiowski O., Bauer A., Friedlmeier T.M., Powalla M. Properties of Cu(In,Ga)Se2 solar cells with new record efficiencies up to 21.7. Phys. Status Solidi. 2015;9(1):28–31. doi: 10.1002/pssr.201409520. [DOI] [Google Scholar]
- 2.Bouich A., Torres J.C., Khattak Y.H., Baig F., Marí-Guaita J., Soucase B.M., et al. Bright future by controlling α/δ phase junction of formamidinium lead iodide doped by imidazolium for solar cells: Insight from experimental, DFT calculations and SCAPS simulation. Surf. Interfaces. 2023;40:103159. [Google Scholar]
- 3.Nakamura M., Yamaguchi K., Kimoto Y., Yasaki Y., Kato T., Sugimoto H. Cd-free Cu(in,Ga)(Se,S)2 thin-film solar cell with record efficiency of 23.35. IEEE J. Photovoltaics. 2019;9(6):1863–1867. doi: 10.1109/JPHOTOV.2019.2937218. [DOI] [Google Scholar]
- 4.Green M.A. Corrigendum to “improved silicon optical parameters at 25°C, 295K and 300K including temperature coefficients” [prog. Photovolt: res. Appl. 2022; 30: 164–179] Prog. Photovolt. 2022;30(9):1144–1145. doi: 10.1002/pip.3560. [DOI] [Google Scholar]
- 5.Tsakalakos L. Nanostructures for photovoltaics. Mater. Sci. Eng. R Rep. 2008;62(6):175–189. doi: 10.1016/j.mser.2008.06.002. [DOI] [Google Scholar]
- 6.Oh Y., Yang W., Kim J., Woo K., Moon J. Aqueous solution-phase selenized CuIn(S,Se)2 thin film solar cells annealed under inert atmosphere. ACS Appl. Mater. Interfaces. 2015;7(40):22570–22577. doi: 10.1021/acsami.5b06996. [DOI] [PubMed] [Google Scholar]
- 7.Uhl A.R., Katahara J.K., Hillhouse H.W. Molecular-ink route to 13.0% efficient low-bandgap CuIn(S,Se)2 and 14.7% efficient Cu(In,Ga)(S,Se)2 solar cells. Energy Environ. Sci. 2016;9(1):130–134. doi: 10.1039/C5EE02870A. [DOI] [Google Scholar]
- 8.Septina W., Kurihara M., Ikeda S., Nakajima Y., Hirano T., Kawasaki Y., Harada T., Matsumura M. Cu(In,Ga)(S,Se)2 thin film solar cell with 10.7% conversion efficiency obtained by selenization of the Na-doped spray-pyrolyzed sulfide precursor film. ACS Appl. Mater. Interfaces. 2015;7(12):6472–6479. doi: 10.1021/am507684x. [DOI] [PubMed] [Google Scholar]
- 9.Babu B.J., Velumani S., Morales-Acevedo A., Asomoza R. Properties of CuInGaSe thin films prepared by chemical spray pyrolysis. 2010. [DOI]
- 10.Aksu S., Pinarbasi M. Electrodeposition methods and chemistries for deposition of CIGS precursor thin films. 2011. [DOI]
- 11.Başol B., Pinarbasi M., Aksu S., Wang J., Matus Y., Johnson T., Han Y., Narasimhan M., Metin B. 23rd European Photovoltaic Solar Energy Conference; 2008. Electroplating-based CIGS Technology for Roll-To-Roll Manufacturing. [Google Scholar]
- 12.Duchatelet A., Sidali T., Loones N., Savidand G., Chassaing E., Lincot D. 12.4% Efficient Cu(In,Ga)Se2 solar cell prepared from one step electrodeposited Cu–In–Ga oxide precursor layer. Sol. Energy Mater. Sol. Cell. 2013;119:241–245. doi: 10.1016/j.solmat.2013.07.053. [DOI] [Google Scholar]
- 13.Bouabid K., Ihlal A., Manar A., Outzourhit A., Ameziane E.L. Effect of deposition and annealing parameters on the properties of electrodeposited CuIn1−xGaxSe2 thin films. Thin Solid Films. 2005;488(1–2):62–67. doi: 10.1016/j.tsf.2005.04.111. [DOI] [Google Scholar]
- 14.Zangari G. Electrodeposition of alloys and compounds in the era of microelectronics and energy conversion technology. Coatings. 2015;5(2):195–218. doi: 10.3390/coatings5020195. [DOI] [Google Scholar]
- 15.Lincot D., Guillemoles J.F., Taunier S., Guimard D., Sicx-Kurdi J., Chaumont A., Roussel O., Ramdani O., Hubert C., Fauvarque J.P. Chalcopyrite thin film solar cells by electrodeposition. Sol. Energy. 2004;77(6):725–737. doi: 10.1016/j.solener.2004.05.024. [DOI] [Google Scholar]
- 16.Calixto M.E., Dobson K.D., McCandless B.E., Birkmire R.W. Controlling growth chemistry and morphology of single-bath electrodeposited Cu(in,Ga)Se[sub 2] thin films for photovoltaic application. J. Electrochem. Soc. 2006;153(6):G521. doi: 10.1149/1.2186764. [DOI] [Google Scholar]
- 17.Kois J., Bereznev S., Mellikov E., Öpik A. Electrodeposition of CuInSe2 thin films onto Mo-glass substrates. Thin Solid Films. 2006;511–512:420–424. doi: 10.1016/j.tsf.2005.11.075. [DOI] [Google Scholar]
- 18.Oliveira M.C.F., Azevedo M., Cunha A. A voltammetric study of the electrodeposition of CuInSe2 in a citrate electrolyte. Thin Solid Films. 2002;405(1–2):129–134. doi: 10.1016/S0040-6090(01)01720-5. [DOI] [Google Scholar]
- 19.Deepa K.G., Lakshmi Shruthi N., Anantha Sunil M., Nagaraju J. Cu(In,Al)Se2 thin films by one-step electrodeposition for photovoltaics. Thin Solid Films. 2014;551:1–7. doi: 10.1016/j.tsf.2013.10.180. [DOI] [Google Scholar]
- 20.Rohom A.B., Londhe P.U., Chaure N.B. The effect of pH and selenization on the properties of CuInSe2 thin films prepared by electrodeposition technique for device applications. J. Solid State Electrochem. 2015;19(1):201–210. doi: 10.1007/s10008-014-2582-0. [DOI] [Google Scholar]
- 21.Sani R., Manivannan R., Noyel Victoria S. One step electrodeposition of copper zinc tin sulfide using sodium thiocyanate as complexing agent. J. Electrochem. Sci. Tech. 2018;9(4) doi: 10.5229/JECST.2018.9.4.308. [DOI] [Google Scholar]
- 22.Frontini M.A., Vázquez M. Electrodeposition of CuInSe2 in citrate-containing electrolytes. J. Mater. Sci. 2010;45(11):2995–3000. doi: 10.1007/s10853-010-4300-3. [DOI] [Google Scholar]
- 23.Arefi-Rad M.R., Kafashan H. Pb-doped SnS nano-powders: comprehensive physical characterizations. Opt. Mater. 2020;105 doi: 10.1016/j.optmat.2020.109887. [DOI] [Google Scholar]
- 24.Alasvand A., Kafashan H. Investigation the effect of Pb incorporation on the surface characterizations of electrodeposited CdSe nanostructures. J. Alloys Compd. 2020;817 doi: 10.1016/j.jallcom.2019.152711. [DOI] [Google Scholar]
- 25.Shafi M.A., Khan L., Ullah S., Bouich A., Ullah H., Mari B. Synthesis of CZTS kesterite by pH adjustment in order to improve the performance of CZTS thin film for photovoltaic applications. Micro Nanostruc. 2022;164 doi: 10.1016/j.spmi.2022.107185. [DOI] [Google Scholar]
- 26.Ananthoju B., Kushwaha A., Sonia F.J., Aslam M. 2013. Structural and Optical Properties of Electrochemically Grown Highly Crystalline Cu[sub 2]ZnSnS[sub 4] (CZTS) Thin Films. [DOI] [Google Scholar]
- 27.Satoh T., Hayashi S., Nishiwaki S., Shimakawa S., Hashimoto Y., Negami T., Uenoyama T. Fabrication of Cu(In,Ga)Se2 by in-line evaporation (composition monitoring method using heat radiation) Sol. Energy Mater. Sol. Cell. 2001;67(1–4):203–207. doi: 10.1016/S0927-0248(00)00282-8. [DOI] [Google Scholar]
- 28.Fiat Varol S., Babür G., Çankaya G., Kölemen U. Synthesis of sol–gel derived nano-crystalline ZnO thin films as TCO window layer: effect of sol aging and boron. RSC Adv. 2014;4(100):56645–56653. doi: 10.1039/C4RA11324A. [DOI] [Google Scholar]
- 29.Valdés M., Vázquez M., Goossens A. Electrodeposition of CuInSe2 and In2Se3 on flat and nanoporous TiO2 substrates. Electrochim. Acta. 2008;54(2):524–529. doi: 10.1016/j.electacta.2008.07.036. [DOI] [Google Scholar]
- 30.Kang F., Ao J.P., Sun G.Z., He Q., Sun Y. Growth and characterization of CuInSe2 thin films via one-step electrodeposition from a lactic acid/sodium lactate buffer system. Mater. Chem. Phys. 2009;115(2–3):516–520. doi: 10.1016/j.matchemphys.2009.02.006. [DOI] [Google Scholar]
- 31.Zhang F., Sun C., Bajracharya C., Rodriguez R.G., Pak J.J. Fabrication and characterization of thin film solar cell made from CuIn0.75Ga0.25S2Wurtzite nanoparticles. J. Nanomater. 2013:1–5. doi: 10.1155/2013/320375. 2013. [DOI] [Google Scholar]
- 32.Singh U.P., Patra S.P. Progress in polycrystalline thin-film Cu(in,Ga)Se2Solar cells. Int. J. Photoenergy. 2010:1–19. doi: 10.1155/2010/468147. 2010. [DOI] [Google Scholar]
- 33.Zhang S.B., Wei S.-H., Zunger A., Katayama-Yoshida H. Defect physics of theCuInSe2chalcopyrite semiconductor. Phys. Rev. B. 1998;57(16):9642–9656. doi: 10.1103/PhysRevB.57.9642. [DOI] [Google Scholar]
- 34.Kumar A. Efficiency enhancement of CZTS solar cells using structural engineering. Superlattice. Microst. 2021;153 doi: 10.1016/j.spmi.2021.106872. [DOI] [Google Scholar]
- 35.Kumar A., Ranjan P. Defects signature in VOC characterization of thin-film solar cells. Sol. Energy. 2021;220:35–42. doi: 10.1016/j.solener.2021.03.017. [DOI] [Google Scholar]
- 36.Movla H. Optimization of the CIGS based thin film solar cells: numerical simulation and analysis. Optik. 2014;125(1):67–70. doi: 10.1016/j.ijleo.2013.06.034. [DOI] [Google Scholar]
- 37.Mostefaoui M., Mazari H., Khelifi S., Bouraiou A., Dabou R. Simulation of high efficiency CIGS solar cells with SCAPS-1D software. Energy Proc. 2015;74:736–744. doi: 10.1016/j.egypro.2015.07.809. [DOI] [Google Scholar]
- 38.Jarosz G., Marczyński R., Signerski R. Effect of band gap on power conversion efficiency of single-junction semiconductor photovoltaic cells under white light phosphor-based LED illumination. Mater. Sci. Semicond. Process. 2020;107 doi: 10.1016/j.mssp.2019.104812. [DOI] [Google Scholar]
- 39.Bouich A., Torres J.C., Chfii H., Marí-Guaita J., Khattak Y.H., Baig F., Soucase B.M., Palacios P. Delafossite as hole transport layer a new pathway for efficient perovskite-based solar sells: insight from experimental, DFT and numerical analysis. Sol. Energy. 2023;250:18–32. doi: 10.1016/j.solener.2022.12.022. [DOI] [Google Scholar]
- 40.Doumbia Y., Bouich A., Soucasse B.M., Soro D. Boosting the stability and growth of methylammonium lead bromide perovskites film doped with FA for solar cells. Opt. Mater. 2023;137 doi: 10.1016/j.optmat.2023.113563. [DOI] [Google Scholar]
- 41.Touré A., Bouich A., Soucasse B.M., Soro D. Investigation of the optoelectronics properties and stability of Formamidinium lead mixed halides perovskite. Opt. Mater. 2023;135 doi: 10.1016/j.optmat.2022.113334. [DOI] [Google Scholar]
- 42.Bouri N., Talbi A., Khaaissa Y., Derbali S., Bouich A., Nouneh K. Insight into MAPb1−xEuxI3 based perovskite solar cell performance using SCAPS Simulator. Optik. 2022;271 doi: 10.1016/j.ijleo.2022.170235. [DOI] [Google Scholar]
- 43.Koné K.E., Bouich A., Soucase B.M., Soro D. Manufacture of different oxides with high uniformity for copper zinc tin sulfide (CZTS) based solar cells. J. Mol. Graph. Model. 2023;121 doi: 10.1016/j.jmgm.2023.108448. [DOI] [PubMed] [Google Scholar]
- 44.Koné K.E., Bouich A., Soro D., Soucase B.M. Insight of ZnO/CuO and ZnO/Cu2O solar cells efficiency with SCAPS simulator. Opt. Quant. Electron. 2023;55(7) doi: 10.1007/s11082-023-04892-9. [DOI] [Google Scholar]
- 45.Doumbia Y., Bouich A., Soro D., Soucase B.M. Mixed halide head perovskites thin films: stability and growth investigation. Optik. 2022;261:169222. [Google Scholar]
- 46.A. Bouich. Study and characterization of hybrid perovskites and copper-indium-gallium selenide thin films for tandem solar cells. 2021. (Doctoral dissertation, Universitat Politècnica de València) [DOI] [Google Scholar]
- 47.N’guessan A.I., Bouich A., Touré A., Soucasse B.M., Soro D. Influence of Sulfur Content in Zn (O, S) Buffer Layer onto Copper Indium Gallium Sulfur-Based Solar Cells Through SurfaceEngineering at ZnO1−xSx/CIGS Interface. 2023. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.