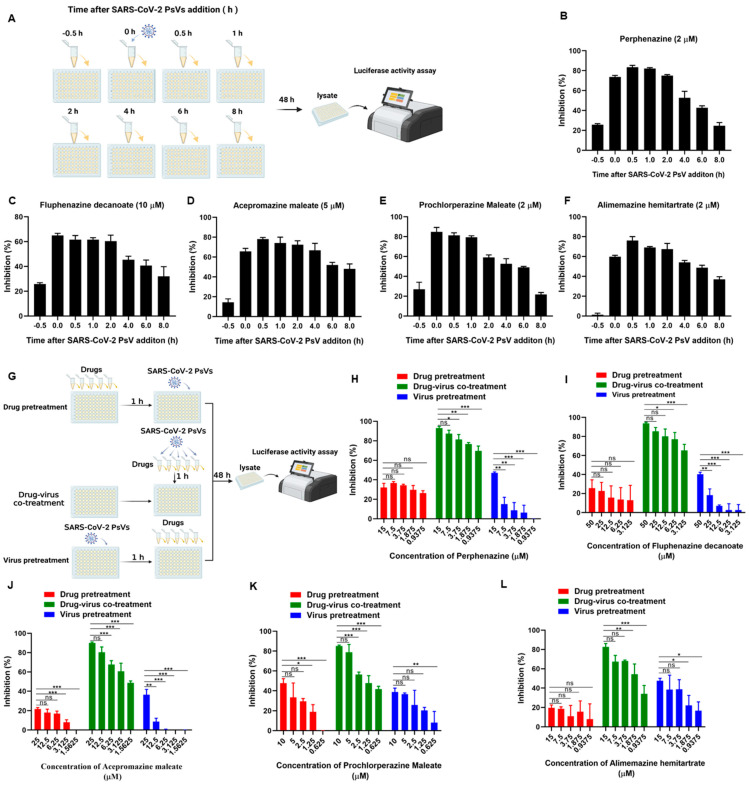
Figure 3.
Phenothiazines inhibited SARS-CoV-2 PsV infection at the early stage. (A) Schematic diagram of time-of-addition assay. HEK293T-hACE2 cells were incubated with SARS-CoV-2 PsV (D614), and the candidate compounds at the indicated concentration were added 0.5 h before or 0, 0.5, 1, 2, 4, 6, or 8 h after the addition of the PsV. The cells were lysed 48 h post-infection to determine the entry inhibition efficacy. (B–F) The inhibitory activities of perphenazine (B), fluphenazine decanoate (C), acepromazine maleate (D), prochlorperazine maleate (E), and alimemazine hemitartrate (F) at the indicated time points before or after SARS-CoV-2 PsV infection. (G) Schematic diagram of the three kinds of treatment assays to determine the target of phenothiazines. Drug pretreatment group: HEK293T-hACE2 cells were pre-treated with different concentrations of compounds for 1 h, and then cells were infected with SARS-CoV-2 PsV. Drug-virus co-treatment group: phenothiazines and SARS-CoV-2 PsV were co-incubated at 37 °C for 1 h and then co-treated the cells at the same time. Virus pretreatment group: HEK293T-hACE2 cells were pre-infected with SARS-CoV-2 PsV for 1 h, and then cells were treated with different concentrations of compounds. (H–L) The inhibitory activities of perphenazine (H), fluphenazine decanoate (I), acepromazine maleate (J), prochlorperazine maleate (K), and alimemazine hemitartrate (L) against SARS-CoV-2 PsV infection when treated with different ways. Samples were tested in triplicate, and the experiments were repeated at least twice. Data are presented in mean ± SD. One-way ANOVA followed by Dennett’s multiple-comparison post hoc test was used to detect differences between the groups. p-values below 0.05 (p < 0.05) were considered to demonstrate statistically significant differences. Statistical significance was defined as * p < 0.05, ** p < 0.01, *** p < 0.001.