Table 8.
FDA-Approved Fluorinated Miscellaneous Drugs.
| Name | Structure | Therapeutic Uses |
|---|---|---|
| Asciminib |
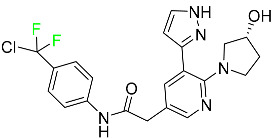
|
Chronic-phase chronic myeloid leukemia (Ph+ CML) |
| Atogepant |
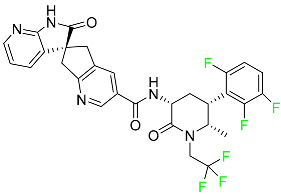
|
Episodic migraine headaches |
| Avacopan |
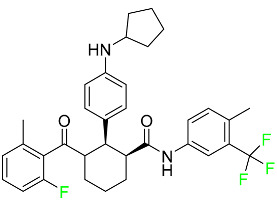
|
Granulomatosis polyangiitis (GPA) or microscopic polyangiitis |
| Delafloxacin |

|
Skin structural infections |
| Elagolix |
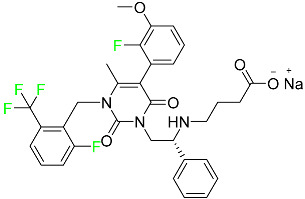
|
Endometriosis-related discomfort |
| Enasidenib |
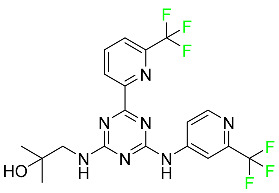
|
Acute myeloid leukemia |
| Lasmiditan |
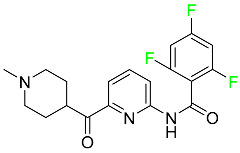
|
Acute migraine headaches with or without aura |
| Lemborexant |
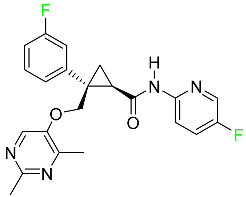
|
Insomnia that interferes with falling asleep and/or staying asleep |
| Lumateperone |

|
Positive and negative symptoms in schizophrenia patients |
| Melphalan flufenamide |
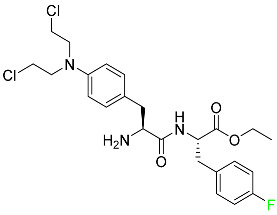
|
Relapsed or resistant multiple myeloma |
| Netupitant |

|
Nausea and vomiting brought on by chemotherapy in addition to other medications |
| Pimavanserin |
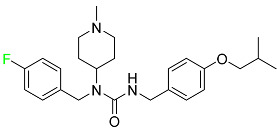
|
Anti-psychotic drug |
| Selinexor |

|
Multiple myeloma |
| Tafenoquine |
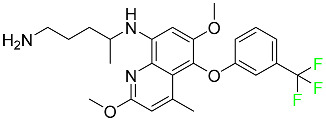
|
Treatment and prevention of vivax malaria |
| Upadacitinib |

|
Moderate to severe forms of rheumatoid arthritis, active psoriatic arthritis, ankylosing spondylitis, and severe atopic dermatitis |
| Ubrogepant |
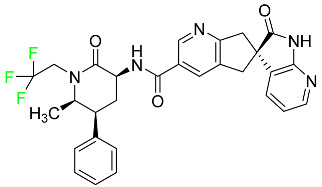
|
Acute migraines both with and without aura |
| Vericiguat |
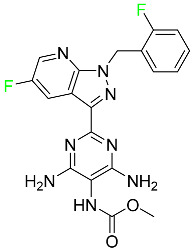
|
Systolic heart failure. |
