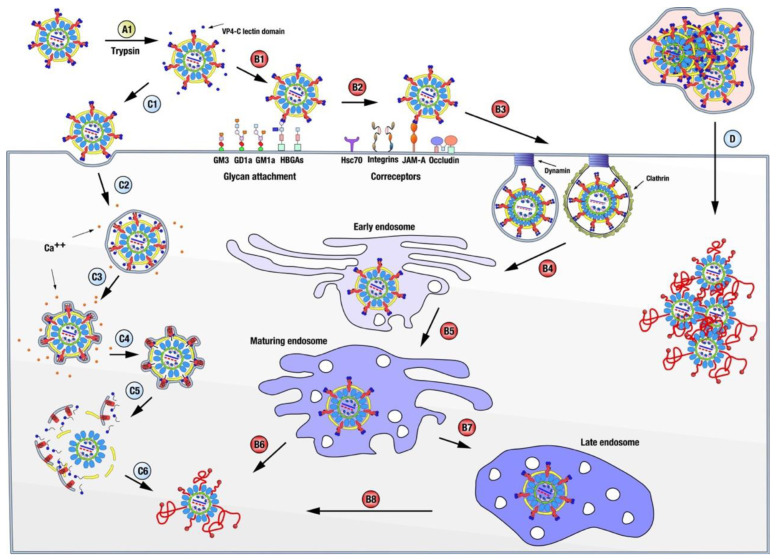
Figure 3.
Rotavirus disassembly. Rotavirus disassembly begins with the cleavage of the outer capsid protein VP4 by trypsin-like proteases (A1), which release the lectin domains of the VP4-C chains from the virus. Rotavirus internalization progresses sequentially through interactions with glycan attachment molecules (B1) and coreceptors (B2), followed by endocytosis, which, depending on the strain, could be clathrin-dependent or independent (B3). Incoming viral particles follow the classical endocytic pathway (B4), reaching maturing endosomes (B5). From here (B6), some strains (termed early-penetration strains) can be released into the cell cytoplasm and initiate transcription. In contrast, late-penetration strains must first reach the late endosome compartment (B7) before they can be released to initiate a productive infection (B8). Our current model for membrane penetration (C1–C6) has been established using the RRV strain in BCS-1 cells and relies primarily on viral components. Initial binding to the cell membrane occurs through the interaction of the distal lectin domains of the two VP8* molecules of chains A and B with a sialylated ganglioside. Following attachment, progressive interactions of the adjacent lectin domains with the cell membrane initiate the invagination of the particle (C1), which is then engulfed in a loose-fitting vesicle (C2). Spontaneous fluctuations lead to dissociation of the lectin domains, revealing the two hydrophobic loops beneath them and triggering their irreversible insertion into the membrane. This is followed by the reorganization of the ß-barrel domains, adopting a trimeric structure in which the hydrophobic loops of the three ß-barrel domains are inserted into the membrane (C3). The free energy released during this process is likely the driving force for the wrapping of the virus in a tight-fitting membrane (C4). Finally, the spikes transition to the reversed conformation, which occurs when the amino acids of the foot domain of each of the three subunits, previously located in the foot cavity, are pulled towards and inserted into the membrane. The insertion of multiple foot domains (C4) leads to perforation (C5) and the release of DLP into the cytoplasm, starting transcription (C6). A third mechanism of entry for rotavirus, vesicle-mediated en bloc transmission, has been recently discovered to play a significant role in transmission [69], but it remains to be characterized further (D).