Abstract
Background:
Many studies have used functional optical zone (FOZ) as a measure to compare different refractive laser treatment modalities. However, to our knowledge, no study has compared wavefront- optimized (WFO) and wavefront-guided (WFG) laser in situ keratomileusis (LASIK) using FOZ. We compared the FOZ after WFO versus WFG LASIK in patients with myopia and myopic astigmatism.
Methods:
In this prospective comparative study, we included 100 myopic eyes of 50 patients with or without astigmatism. They were divided into two groups according to the platform used: WFO or WFG femtosecond LASIK. Using Holladay’s equivalent keratometry reading (EKR) report of Pentacam HR, FOZ was defined as a zone centered on the pupil center with a standard deviation (SD) of 0.5 D, around the mean EKR. The differences in FOZ between the two platforms were analyzed at 3 months postoperatively. Visual acuity, refractive error, corneal asphericity (Q-value), and root mean square of higher-order aberrations (RMS for HOAs) were evaluated and compared.
Results:
The mean (SD) of patient age was 26.64 (5.67) years. The preoperative characteristics of the two groups were comparable (all P > 0.05). The intended optical zone (IOZ) was 6 mm in both groups. The mean laser ablation depth was significantly greater in the WFG group (18 µm per D) than in the WFO group (16 µm per D) (P = 0.035). At 3 months postoperatively, the mean (SD) of FOZ diameter was 4.32 (0.94) mm (71.99% [15.68%] of intended optical zone) in the WFO group and 4.16 (1.13) mm (69.33% [18.78%] of intended optical zone) in the WFG group, with no significant difference between the two groups (P = 0.622). The change in corneal asphericity was greater in the WFG group than in the WFO group (P = 0.034). Postoperative mean corrected and uncorrected distance visual acuity, manifest refraction, and RMS for HOAs showed no significant difference between the two groups (all P > 0.05).
Conclusions:
We found that WFG LASIK resulted in greater ablation depth and change in corneal asphericity than WFO LASIK at 3 months postoperatively. However, there was no significant difference in FOZ diameter, refractive error, and RMS for HOAs between the two groups. Further research is needed to confirm these findings.
Key Words: LASIK, laser in situ keratomileusis, wavefront-guided, wavefront-optimized, functional optical zone, intended optical zone, higher order aberrations, corneal asphericity
INTRODUCTION
Conventional laser refractive surgery is effective for correcting simple spherocylindrical refractive errors. However, it changes corneal shape from a prolate to a more oblate profile, leading to greater higher-order aberrations (HOAs) and lower contrast sensitivity [1, 2]. Wavefront-optimized (WFO) and wavefront-guided (WFG) platforms have been developed to compensate for this phenomenon [3]. The former applies more ablation to the periphery, considering the eye’s refractive error and preoperative keratometry reading, while the latter offers a customized treatment plan that reduces both preoperative and postoperative HOAs. Many studies have shown that the two platforms are largely equivalent [4-7].
Not all intended optical zones (IOZs) provide functional vision [8, 9]. According to Wachler et al., the functional optical zone (FOZ) is defined as the central part of the cornea with a high level of optical quality and fewer aberrations that could produce a potential visual acuity of 20/32 [10, 11]. Many studies have used FOZ as a measure to compare different refractive laser treatment modalities [5, 6, 12-14]. Many methods have been suggested for its measurement; however, topography-based methods are the most practical because of their ease of implementation and direct spatial correspondence to corneal topographic maps [15].
Here, we aimed to compare the FOZ after WFO versus WFG LASIK for correction of myopia with or without astigmatism. The primary outcome measures were differences in FOZ between the two platforms, and the secondary outcome measures were uncorrected distance visual acuity (UCDVA), HOAs, and changes in corneal asphericity at 3 months postoperatively.
METHODS
In this prospective comparative study, we included 100 myopic eyes of 50 patients with or without astigmatism who underwent femtosecond LASIK using either the WFO or the WFG platform from June 2018 to December 2020. Ethical approval was obtained from the Ethical Review Committee of the Ain Shams University Faculty of Medicine, Cairo, Egypt. In accordance with the ethical standards stated by the Faculty of Medicine Ain- Shams University and good clinical practice, written consent for participation and publication for the study was obtained prior to surgery.
We included patients ages 20 to 37 years who had stable refractive status (a change of < 0.50 D in the spherical or cylindrical component over at least one year), a best-corrected distance visual acuity (BCDVA) 20/20 using Snellen’s visual acuity chart, myopia (range: -1.00 D to -6.00 D) with or without myopic astigmatism (range: 0.00 D to -3.00 D), a healthy cornea, a mesopic pupil diameter ≤ 6 mm, and corneal thickness ≥ 500 µm (with an estimated residual stromal bed thickness > 300 µm). Patients with unstable refraction, hyperopia, myopia greater than -6.00 D and astigmatism greater than -3.00 D, eyelid abnormalities, moderate to severe meibomian gland dysfunction, corneal opacities, corneal basement membrane dystrophies, a history of herpes keratitis, a suspicious corneal topography, ectatic corneal diseases (i.e., keratoconus, forme fruste keratoconus, or pellucid marginal degeneration), diabetes mellitus, collagen vascular diseases, an immunocompromised status, autoimmune diseases, and those who were pregnant or lactating or taking medications such as antihypertensives (e.g., propranolol), isotretinoin, oral contraceptive pills, and psychiatric medications were excluded.
The preoperative evaluation included medical (including ophthalmic), medication, and family history, and a history of using spectacles or contact lenses. Contact lenses were removed (7 days for soft non-toric, 14 days for soft toric, and 1 month for every decade of use for rigid gas-permeable contact lenses) prior to the preoperative evaluation. UCDVA and BCDVA were measured using Snellen’s chart and expressed as decimal values. Manifest and cycloplegic refraction were measured using an autorefractometer (ARK-1 Auto Ref/Keratometer; Nidek Co., Ltd., Japan). Slit lamp examination was performed with an SL-3G slit lamp (Topcon Co., Tokyo, Japan), followed by fundus biomicroscopy with a noncontact lens +90 D (Volk Optical Inc., Mentor, OH, USA) and applanation tonometry with a Goldmann tonometer (AT 900 C/M; Haag- Streit, Bern, Switzerland) after obtaining axial/sagittal curvature maps, pachymetry maps, corneal surface asphericity, pupil size, and corneal regularity indices (index of surface variance [ISV] and index of height decentration [IHD]) using a Pentacam HR (Oculus Optikgerate GmbH, Wetzlar, Germany).
Patients were divided into two groups according to the platform used for LASIK: WFO or WFG. All had a fied IOZ of 6 mm. WFO LASIK was performed at the Watany Eye Hospital, Cairo, Egypt (by Elraggal T.). LASIK flaps were made with a WaveLight® FS200 laser (Alcon Laboratories, Inc., Fort Worth, TX, USA), and ablation was performed using the WaveLight® EX500 excimer laser system (Alcon, Inc., Huenberg, Switzerland). WFG LASIK was performed at Magrabi Eye Hospital, Cairo, Egypt (by Seleet M.). All eyes underwent preoperative wavefront analysis using the VISX CustomVue WaveScan aberrometer v.3.62 (Fourier) (Advanced Medical Optics, Santa Ana, CA, USA). LASIK flaps were made with IntraLase iFS ( Johnson & Johnson Inc., Santa Ana, CA, USA), and ablation was performed using the VISX CustomVue™ STAR S4 IR™ Ecimer Laser ( Johnson & Johnson Inc., Santa Ana, CA, USA) with Active-Track™ iris registration.
Postoperatively, all patients were prescribed topical dexamethasone 0.1% + tobramycin 0.3% (Tobradex; Alcon, Fort Worth, TX, USA) eye drops 6 times/day for 1 week, plus artificial tears (Systane® Ultra Lubricant Eye Drops; Alcon, Fort Worth, TX, USA) 6 times/day for 1 month. Routine follow-up visits were performed the next day and at 1 week and 3 months postoperatively. In the last follow-up visit at 3 months, we measured UCDVA and BCDVA using Snellen’s chart and expressed as decimal values. Manifest and cycloplegic refraction were measured. Intraocular pressure was checked using air‐puff tonometry (Topcon CT‐80 Computerized Auto Tonometer; Topcon, Tokyo, Japan), and slit lamp biomicroscopy was used for anterior segment examination to ensure the flap was in place and to look for any signs of late-onset complications (e.g., dry eye and ectasia). Fundus biomicroscopy was performed with a Volk 90D lens after pupillary dilatation. Aial/sagittal curvature maps, pachymetry maps, corneal surface asphericity, and corneal regularity indices were obtained using Pentacam HR. FOZ and corneal front surface wavefront aberrations such as root mean square of higher-order aberrations (RMS for HOAs) and spherical aberration Zernike coefficient (Z40) were measured and compared between the two groups using tomography-based Scheimpflug imaging (Pentacam HR).
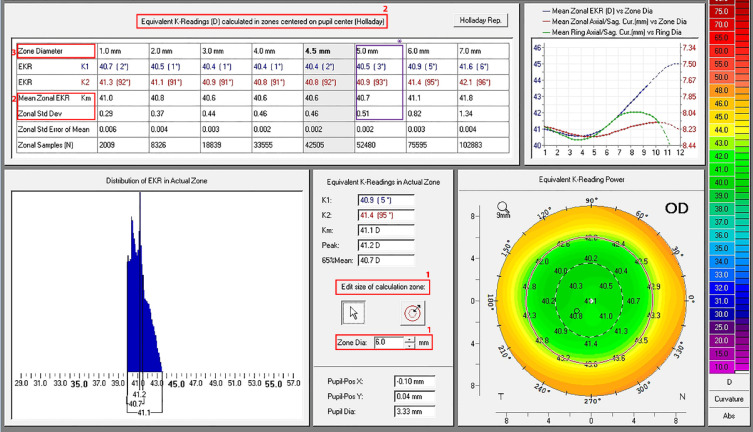
To measure the FOZ, we chose Holladay’s equivalent keratometry reading (EKR) report (power map) of Pentacam HR. Our aim was to determine the area in the central cornea with uniform refractive power (± 0.5 D), indicative of an effective treatment [11]. The measurement steps were as follows. (1) We set the size of the calculation zone at a zonal diameter of 6 mm. (2) We chose Holladay zone centered on the pupil center with a zonal standard deviation (SD) nearest to 0.5 D around the mean EKR. This threshold value of 0.5 D was chosen, as UCDVA of 20/32, which results from a -0.5 D defocus, does not interfere with daily life activities [10, 14, 16]. (3) We recorded the Holladay zonal diameter that met these criteria as the diameter of FOZ (Figure 1).
Figure 1.
A sample Pentacam HR image showing how the functional optical zone (FOZ) was measured postoperatively. (1) The size of the calculation zone was set at a zonal diameter of 6 mm. (2) The Holladay zone was centered on the pupil center with a zonal standard deviation (SD) nearest to 0.5 D around the mean EKR [10, 14, 16]. Finally, the Holladay zonal diameter that met these criteria was recorded as the diameter of FOZ. Here, when Zonal diameter set to 6 mm (1), the mean EKR of 40.7 mm with an SD of 0.5 D (2) showing a FOZ diameter of 5 mm (3).
Data were gathered, revised, coded, and uploaded to the Statistical Package for the Social Sciences (IBM SPSS Statistics for Windows, version 23.0; IBM Corp., Armonk, NY, USA). Comparisons between groups were performed using the independent t-test and the Mann–Whitney test. The Kolmogorov–Smirnov test was used to assess the normality of the data, and Spearman’s rank correlation coefficient was used to calculate the correlation. The confidence level was set at 95%, and the accepted margin of error was set at 5%. Statistical significance was set at P < 0.05, and a P < 0.01 was considered to be highly significant.
RESULTS
We included 50 patients with a mean (SD) age of 26.64 (5.67) years. Twenty-three (46%) were men and 27 (54%) were women. As shown in Table 1, preoperative and intraoperative parameters were comparable between the two groups (all P > 0.05), except for the mean maximal laser ablation depth, which was significantly greater in the WFG group than in the WFO group (P = 0.035). All surgeries were uneventful with an IOZ of 6 mm, a flap diameter ranging from 8.7 to 9 mm, and a flap thickness ranging from 90 to 120 µm (Table 1).
Table 1.
Preoperative and intraoperative parameters in the WFO and WFG LASIK groups
| Preoperative data | Group A ( n = 50) | Group B ( n = 50) | P -value |
|---|---|---|---|
| UCDVA (decimal), Mean ± SD (Range) | 0.44 ± 0.13 (0.2 to 0.7) | 0.47 ± 0.14 (0.2 to 0.7) | 0.426 |
| BCDVA (decimal), Mean ± SD (Range) | 0.99 ± 0.03 (0.9 to 1) | 1.00 ± 0.02 (0.9 to 1) | 0.143 |
| Sphere (D), Mean ± SD (Range) | -3.84 ± 1.28 (-6 to -1.75) | -4.14 ± 1.42 (-6 to -1.75) | 0.242 |
| Cylinder (D), Mean ± SD (Range) | -1.24 ± 1.00 (-3 to 0.00) | -0.84 ± 0.44 (-1.75 to -0.25) | 0.213 |
| SE (D), Mean ± SD (Range) | -4.45 ± 1.48 (-7.5 to -2.25) | -4.56 ± 1.39 (-6.63 to -2.13) | 0.400 |
| K1 (D), Mean ± SD (Range) | 43.03 ± 1.74 (39.61 to 45.9) | 42.27 ± 1.69 (39.5 to 45.8) | 0.079 |
| K2 (D), Mean ± SD (Range) | 44.35 ± 1.68 (40.8 to 46.87) | 43.29 ± 1.53 (40.5 to 46.3) | 0.056 |
| Kmean (D), Mean ± SD (Range) | 43.70 ± 1.66 (40.5 to 46.1) | 42.79 ± 1.58 (40 to 46) | 0.061 |
| Kmax (D), Mean ± SD (Range) | 44.78 ± 1.57 (41.2 to 46.95) | 43.77 ± 1.56 (40.6 to 46.9) | 0.091 |
| Pachy apex (µm), Mean ± SD (Range) | 570.04 ± 41.66 (524 to 648) | 566.32 ± 28.57 (523 to 619) | 0.594 |
| Thinnest location (µm), Mean ± SD (Range) | 565.46 ± 41.56 (520 to 643) | 562.10 ± 29.44 (514 to 616) | 0.604 |
| ISV, Mean ± SD (Range) | 18.36 ± 3.70 (13 to 26) | 15.56 ± 2.54 (11 to 21) | 0.642 |
| IHD, Mean ± SD (Range) | 0.007 ± 0.003 (0.004 to 0.015) | 0.008 ± 0.004 (0.003 to 0.014) | 0.063 |
| Q value, Mean ± SD (Range) | -0.25 ± 0.03 (-0.32 to -0.21) | -0.26 ± 0.05 (-0.38 to -0.16) | 0.058 |
| PD (mm), Mean ± SD (Range) | 2.78 ± 0.32 (2.32 to 3.89) | 3.29 ± 0.57 (2.36 to 4.4) | 0.056 |
| Intraoperative data |
Group A
n = 50 |
Group B
n = 50 |
P -value |
| Flap thickness (µm), Mean ± SD (Range) | 110.80 ± 4.88 (100 to 120) | 90.00 ± 0.00 (90 to 90) | 0.051 |
| Flap diameter (mm), Mean ± SD (Range) | 8.76 ± 0.09 (8.7 to 9) | 8.85 ± 0.14 (8.7 to 9) | 0.053 |
| Side cut angle (degree), Mean ± SD (Range) | 120.00 ± 0.00 (120 to 120) | 120.00 ± 0.00 (120 to 120) | 0.642 |
| IOZ (mm), Mean ± SD (Range) | 6.00 ± 0.00 (6 to 6) | 6.00 ± 0.00 (6 to 6) | 0.542 |
| MAD (µm), Mean ± SD (Range) | 71.80 ± 21.65 (42.05 to 118) | 81.81 ± 24.97 (38 to 118.8) | 0.035 |
| RST(µm), Mean ± SD (Range) | 373.44 ± 33.72 (345 to 460) | 370.14 ± 39.46(324 to 468) | 0.654 |
Abbreviations: n, number; UCDVA, uncorrected distance visual acuity; BCDVA, best-corrected distance visual acuity; SD, standard deviation; sphere, spherical component of manifest refraction; cylinder, cylindrical component of manifest refraction; SE, spherical equivalent of manifest refraction; D, diopter; K1, the steepest corneal meridian; K2, the fiattest corneal meridian; Kmean, mean keratometric power; Kmax, maximum keratometric power; Pachy apex, corneal thickness at the apex; µm, micrometer; thinnest location, thinnest point over the anterior corneal surface; ISV, index of surface variance; IHD, index of height decentration; Q-value, corneal asphericity; PD, pupil diameter; mm, millimeter; IOZ, intended optical zone; MAD, maximal ablation depth; RST, residual stromal thickness; LASIK, laser in situ keratomileusis. Values of P < 0.05 are shown in bold. Note: K1, K2, Kmean, Kmax, pachy apex, thinnest location, ISV, IHD, Q-value, and pupil diameter were measured using Pentacam HR (Oculus Optikgerate GmbH, Wetzlar, Germany). Group A, wavefront-optimized (WFO); Group B, wavefront- guided (WFG).
Outcomes for the last follow-up visit at 3 months are illustrated in Table 2. Changes in corneal asphericity (ΔQ) were significantly greater in the WFG group than in the WFO group (P = 0.034) (Table 2). The UCDVA, BCDVA, spherical equivalent of manifest refraction (SE), maximum keratometric power (Kmax), thinnest location, RMS for HOAs, and Z40 were not different between the two groups (all P > 0.05) (Table 2) and neither were FOZ measurements (P = 0.622) (Table 3). A positive correlation was found between FOZ diameter and postoperative corneal thickness (r = + 0.295; P = 0.038 for WFO, r = + 0.487; P < 0.001 for WFG) and a negative correlation between FOZ diameter and maximal laser ablation depth (r = - 0.626; P < 0.001 for WFO, r = - 0.475; P < 0.001 for WFG), and RMS for HOAs (r = - 0.533; P < 0.001 for WFO, r = - 0.506; P < 0.001 for WFG).
Table 2.
Outcomes at 3 months after WFO or WFG LASIK
| Postoperative data | Group A ( n = 50) | Group B ( n = 50) | P -value |
|---|---|---|---|
| UCDVA (decimal), Mean ± SD (Range) | 0.94 ± 0.09 (0.8 to 1) | 0.96 ± 0.08 (0.8 to 1) | 0.236* |
| BCDVA (decimal), Mean ± SD (Range) | 1.00 ± 0.00 (1 to 1) | 1.00 ± 0.00 (1 to 1) | 1.000* |
| SE (D), Median (IQR), (Range) | 0 (-0.25 to 0), (-0.5 to 0.00) | 0 (0 to 0), (- 0.5 to 0.00) | 0.137** |
| K1, Mean ± SD (Range) | 39.38 ± 2.11 (36 to 43.3) | 38.45 ± 1.50 (35.9 to 41.2) | 0.013* |
| K2, Mean ± SD (Range) | 39.93 ± 2.06 (36.3 to 43.8) | 39.03 ± 1.47 (36.6 to 41.7) | 0.013* |
| Kmax, Mean ± SD (Range) | 43.31 ± 1.53 (40.5 to 46) | 43.12 ± 2.10 (39 to 47) | 0.602* |
| Thinnest Location, Mean ± SD (Range) | 481.34 ± 44.86 (409 to 574) | 480.14 ± 39.46 (434 to 578) | 0.887* |
| RMS for HOA, Median (IQR), (Range) | 0.69 (0.6 to 0.88), (0.47 to 1.49) | 0.7 (0.58 to 0.89), (0.29 to 1.21) | 0.863** |
| SA (Z40), Mean ± SD (Range) | 0.53 ± 0.11 (0.23 to 0.81) | 0.55 ± 0.20 (0.24 to 0.93) | 0.578* |
| ΔQ value (postoperative -postoperative) , Mean ± SD | 0.79 ± 0.42 | 0.96 ± 0.37 | 0.034* |
Abbreviations: n, number; UCDVA, uncorrected distance visual acuity; BCDVA, best-corrected distance visual acuity; SD, standard deviation; SE, spherical equivalent; D, diopter; IQR, interquartile range; K1, steepest corneal meridian; K2, fiattest corneal meridian; Kmax, maximum keratometric power; Thinnest location, thinnest point over the anterior corneal surface; RMS for HOA, root mean square for higher-order aberrations; SA (Z40), spherical aberration Zernike coefficient Z40; ΔQ value, change in corneal asphericity; LASIK, laser in situ keratomileusis. Values of P < 0.05 are shown in bold. *Independent t-test; **Mann–Whitney test. Note: Group A, wavefront-optimized (WFO); Group B, wavefront-guided (WFG).
Table 3.
FOZ and IOZ changes after WFO or WFG LASIK
| Variable | Group A ( n = 50) | Group B ( n = 50) | P- v a lue * |
|---|---|---|---|
| FOZ diameter (mm), Mean ± SD (Range) | 4.32 ± 0.94 (3 to 6) | 4.16 ± 1.13 (3 to 6) | 0.622 |
| Mean zonal EKR (D), Mean ± SD (Range) | 38.21 ± 1.55 (34.99 to 41.04) | 39.78 ± 2.08 (36.2 to 43.8) | < 0.001 |
| IOZ Changes (mm), Mean ± SD (Range) | 1.68 ± 0.94 (0 to 3) | 1.84 ± 1.13 (0 to 3) | 0.254 |
| FOZ % achieved, Mean ± SD (Range) | 71.99 ± 15.68 (50 to 100) | 69.33 ± 18.78 (50 to 100) | 0.443 |
Abbreviations: FOZ, functional optical zone; SD, standard deviation; EKR, Holladay Equivalent K Reading; D, diopter; IOZ, intended optical zone; IOZ changes, IOZ – FOZ; LASIK, laser in situ keratomileusis. Values of P < 0.05 are shown in bold. Independent t-test. Note: Group A, wavefront-optimized (WFO); Group B, wavefront-guided (WFG).
DISCUSSION
There was no significant difference between the WFO and WFG LASIK groups in terms of FOZ size, refractive outcomes, RMS for HOAs, and spherical aberration (Zernike coefficient Z40) at 3 months postoperatively. The mean maximal laser ablation depth and change in corneal asphericity (ΔQ) were significantly greater in the WFG group than in the WFO group.
Many studies have compared the efficacy of WFO and WFG platforms for myopic correction and concluded that the two yield largely equivalent outcomes [4, 17]. These studies are summarized in Table 4 [3-6, 17-24]. FOZ has also been used as an effective tool for the assessment and comparison of different laser refractive surgery modalities [8, 9]. However, to our knowledge, no study has compared WFG and WFO LASIK using FOZ.
Table 4.
Summary of studies comparing WFO versus WFG LASIK for myopic correction
| Original Papers | |||
|---|---|---|---|
| Author (Year of Publication) | Patients | Meth odology | Conclusions |
| Padmanabhan et al. (2008) [ 18 ] | Fifty-four eyes of 27 patients with myopia or myopic astigmatism. |
Prospective WFO LASIK in 1 eye and WFG LASIK in the fellow eye. One month of follow-up. |
Both WFG and WFO LASIK gave excellent refractive correction results. WFG induced less HOAs and was associated with better contrast sensitivity. |
| Perez-Straziota et al. (2010) [ 6 ] | One hundred thirty-four eyes of patients with myopia or myopic astigmatism. |
Retrospective Sity-si eyes (33 patients) in the WFG group and 66 eyes (45 patients) in WFO. |
No differences in UCDVA, BCDVA, or HOAs between groups (all P > 0.05). |
| Moshirfar et al. (2011) [ 4 ] | Forty-four eyes of 22 patients with myopia with or without astigmatism. |
Prospective, randomized WFO LASIK in 1 eye and WFG LASIK in the fellow eye. Three months of follow-up. |
WFO and WFG had equal visual and safety outcomes. WFO had a significant increase in total RMS for HOAs, and WFG showed a decreasing trend in HOA values. |
| Miraftab et al. (2011) [ 5 ] | Eighty-two eyes of 41 patients with myopia (-7 D) or myopic astigmatism (3 D). |
WFO LASIK in 1 eye and WFG LASIK in the fellow eye. Three months of follow-up. |
HOAs, especially SAs, increased in the same amount in both groups. No significant difference in SE between groups. |
| Sáles et al. (2013) [ 19 ] | Seventy-two eyes of 36 patients with myopia with or without astigmatism. |
Randomized, prospective WFO LASIK in 36 eyes and WFG LASIK in 36 eyes. Twelve months of follow-up. |
WFO and WFG had similar UCDVA outcomes. Contrast sensitivity, astigmatism, coma, and RMS for HOAs showed no significant difference between groups (all P > 0.05). |
| Sáles et al. (2014) [ 20 ] | Twenty-two eyes of 11 patients with hyperopia with or without astigmatism. |
Randomized, prospective WFO LASIK in 11 eyes and WFG LASIK in 11 eyes. Twelve months of follow-up. |
No significant difference was found between the groups in refractive error, predictability, stability, efficacy, safety, contrast sensitivity, and wavefront aberrometer outcomes (coma, trefoil, and RMS for HOAs) (all P < 0.05). |
| He et al. (2014) [ 17 ] | One hundred ten eyes of 55 patients with myopia with or without astigmatism. |
WFO LASIK in 1 eye and WFG LASIK in the fellow eye. Twelve months of follow-up. |
WFO and WFG safely and effectively corrected myopia with or without astigmatism with no significant differences in residual astigmatism or HOAs. WFG offered significant advantages in residual refractive error, UCDVA, and contrast sensitivity. |
| Khalifa et al. (2017) [ 21 ] | Two hundred twenty-one eyes with low (-0.5 to -3 D) and moderate myopic (-3 to -6 D) astigmatism. |
Randomized, prospective WFO LASIK in 112 eyes (54/58, low/moderate myopia), and WFG LASIK 109 eyes (45/64, low/ moderate myopia). Si months of follow-up. |
Significantly better postoperative UCDVA in WFG (P ≤ 0.041). Postoperative SE and magnitude of cylinder were significantly higher in WFO and fewer HOAs were found in WFG . |
| Lee et al. (2018) [ 22 ] | One hundred two eyes of 51 patients with myopia (< -12 D) with or without astigmatism. |
WFO LASIK in 1 eye and WFG LASIK in the fellow eye. Twelve months of follow-up. ΔK/ ΔSE compared using scanning-slit topography (Orbscan; Bausch & Lomb). |
Both WFO and WFG showed no significant difference in change in corneal curvature for a given degree of refractive error on scanning-slit topography to measure ΔK/ΔSE (the ratios of change in simulated K to change in SE). |
| Roe et al. (2019) [ 23 ] | Two hundred eyes of 100 patients with myopia or compound myopic astigmatism. |
Prospective WFO LASIK in 1 eye and WFG LASIK in the fellow eye. Twelve months of follow-up. |
Both types had excellent postoperative UCDVA results, without a significant difference in mean UCDVA. WFG had better contrast sensitivity than WFO. |
| Review Papers | |||
| Author (Year of Publication) | Included studies | Conclusion | |
| Feng et al. (2011) [ 3 ] | Seven articles describing a total of 930 eyes. | No significant difference between groups regarding UCDVA, manifest refraction, SE, or HOAs. WFG had significantly better postoperative aberration profile than WFO with a preoperative RMS for HOAs > 0.3 µm. |
|
| Stonecipher et al. (2018) [ 24 ] | Previous studies in literature used to formulate a decision tree, and 1042 eyes over one year that underwent WFO, WFG, topography modified, and topography-guided laser surgery. |
In the presence of good visual acuity, good night vision, and good visual quality, regular topography, or RMSh < 0.4, the recommendation is to have WFO LASIK; otherwise, WFG. If wavefront measurement is not possible in the absence of good visual acuity, good night vision, and good visual quality, the recommendation is to have topography-guided LASIK. |
|
Abbreviations: LASIK, laser in situ keratomileusis WFO, wavefront-optimized; WFG, wavefront-guided; HOAs, higher order aberrations; UCDVA, uncorrected distance visual acuity; BCDVA, best corrected distance visual acuity; D, diopter; SA, spherical aberration; SE, spherical equivalent; RMS, root mean square; ΔK/ΔSE, change in keratomery/change in spherical equivalent; K: keratometry.
In our study, tomography-based Scheimpflug imaging (Pentacam HR) was chosen to measure FOZ, as done by Hou et al. [13] and Ding et al. [14]; however, our data were collected from Holladay’s detailed report, not from the tangential curvature difference map or the total corneal refractive power (TCRP) map. We used a change of corneal powers within the ± 0.5 D range around the mean EKR. Racine et al. [25] used ± 0.5 D change of powers from the pupil center, Tabernero et al. [15] used a ± 0.5 D range for the statistical mode, and Ding et al. [14] used points that reached a corneal apex refractive power of + 0.5 D. In our method, FOZ measurements were directly obtained from the scans without the need to use formulas and calculations.
In our study, following WFG LASIK, the mean FOZ diameter was 4.16 mm, representing 69.33% of IOZ (P < 0.05). This result was similar to that of Racine et al. [25], where following CustomVue LASIK, mean FOZ diameters (long and short axes) were 5.35 mm and 4.26 mm when compared to the programmed OZ diameters of 6.39 mm and 6 mm, representing 59.58% of the laser-programmed OZ (P < 0.0001) [25]. Concerning WFO ablation, our study showed a decrease in the FOZ (mean diameter of 4.32 mm compared to the IOZ of 6 mm), with a mean difference of -1.68 mm between them (P < 0.05). This was different from the results of Danasoury et al. [11], where optimized prolate ablation (NIDEK laser platform) with a programmed OZ (6.80 mm) resulted in a larger FOZ with a mean difference between its horizontal diameter and the programmed OZ of + 0.377 mm [11]. This discrepancy may be due to a different method (corneal topography axial map with the Klyce–Smolek scale and color scheme) that was used to manually measure FOZ with a 1.50 D change from the central corneal power [26]. In our study, both LASIK platforms (WFO and WFG) yielded a decrease in FOZ size when compared with IOZ, which was similar to results reported after conventional myopic LASIK surgery by Holladay and Janes [12] and Partal and Manche [27]. In our study, the difference in FOZ between WFO and WFG was not significant (P = 0.622). In our study, both groups demonstrated a significant negative correlation between FOZ diameter and maximal laser ablation depth and a significant positive correlation between FOZ diameter and postoperative corneal thickness (all P < 0.05). These findings confirmed the results of Boer Wachler et al. [10] and El Danasoury et al. [11], but were unlike those of Racine et al. [25], who showed that FOZ did not decrease significantly with increasing amount of refractive correction. This controversy may be attributed to different ways of measuring FOZ, with the actual shape of most measured zones not fulfilling an exact ellipse.
Concerning the visual outcomes, both groups in our study achieved excellent refractive outcomes with no significant difference between them. This agrees with the results of Perez-Straziota et al. [6], Meidani and Tzavara [28], Hassan et al. [29], and Mahmoud et al. [30]. On the other hand, our results were different from those reported by He et al. [17], Ghoneim et al. [31], and Roe and Manche [23], where WFG appeared to be superior to WFO (P = 0.016). This can be explained by the contralateral-eye design, which eliminates confounding factors specific to individual patients, such as wound healing and corneal mechanical properties. In our study, postoperative RMS for HOAs and spherical aberration Zernike coefficient (Z40) showed no significant differences between the two groups. This was also observed previously by Roe and Manche [23], He et al. [17], Reed et al. [32], and Mahmoud et al. [30]. However, Moshirfar et al. [4], Khalifa et al. [21], and Ghoneim et al. [31] found that the level of induced HOAs was significantly higher in the WFO group than in the WFG group, which could be attributed to their use of high-resolution aberrometer measurements to define WFG ablation profiles [4, 21, 31]. Concerning the change in corneal asphericity, our results were similar to those of Bottos et al. [33] and Molchan et al. [34], where ΔQ was significantly greater in the WFG group than in the WFO group (P = 0.034). The mean maximal laser ablation depth in our study was greater in the WFG group than in the WFO group (P = 0.035). This finding is consistent with that reported by He et al. [17].
To our knowledge, our study is the first to compare WFG and WFO LASIK using FOZ. There was no significant difference between the two groups. The limitations of this study included a relatively small number of patients, excluding myopes with more than 6 D of refractive error, a short follow-up period, and interoperator variability between both groups. We recommend a contralateral-eye design and larger-scale studies with longer follow-up periods to confirm our results. Further studies are needed to develop a universal method for measuring topographic FOZ.
CONCLUSIONS
We found that WFG LASIK resulted in greater ablation depth and change in corneal asphericity than WFO LASIK at 3 months postoperatively. However, there was no significant difference in FOZ diameter, refractive error, and RMS for HOAs between the two groups. Further research is needed to confirm these findings.
ETHICAL DECLARATIONS
Ethical approval:
Ethical approval was obtained from the Ethics Review Committee of Ain Shams University, Cairo, Egypt. In accordance with the ethical standards stated by the Faculty of Medicine Ain-Shams University and good clinical practice, written consent for participation and publication for the study was obtained prior to surgery.
Conflict of interest:
None.
FUNDING
None.
ACKNOWLEDGMENTS
This work is part of a project entitled “Comparison between wavefront-guided and wavefront-optimized LASIK with regards to the functional optical zone.”
References
- 1.Padmanabhan P, Basuthkar S, Joseph R. Ocular aberrations after wavefront optimized LASIK for myopia. Indian Journal of Ophthalmology. 2010;58(4):307–12. doi: 10.4103/0301-4738.64139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chalita M, Chavala S, Xu M, Krueger RR. Wavefront analysis in post-LASIK eyes and its correlation with visual symptoms, refraction, and topography. Ophthalmology. 2004;111(3):447–53. doi: 10.1016/j.ophtha.2003.06.022. [DOI] [PubMed] [Google Scholar]
- 3.Feng Y, Yu J, Wang Q. Meta-Analysis of Wavefront-Guided vs Wavefront-Optimized LASIK for Myopia. Optometry and Vision Science. 2011;88(12):1463–9. doi: 10.1097/OPX.0b013e3182333a50. [DOI] [PubMed] [Google Scholar]
- 4.Moshirfar M, Betts, Churgin, Maylon H, Neuffer M, Sikder, et al. A prospective, randomized, fellow eye comparison of WaveLight® Allegretto Wave® Eye-Q versus VISX CustomVue™ STAR S4 IR™ in laser in situ keratomileusis (LASIK): analysis of visual outcomes and higher order aberrations. Clinical Ophthalmology. 2011;5:1339–47. doi: 10.2147/OPTH.S24316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miraftab M, Seyedian MA, Hashemi H. Wavefront-Guided vs Wavefront-Optimized LASIK: A Randomized Clinical Trial Comparing Contralateral Eyes. Journal of Refractive Surgery. 2011;27(4):245–50. doi: 10.3928/1081597X-20100812-02. [DOI] [PubMed] [Google Scholar]
- 6.Perez-Straziota CE, Randleman BJ, Stulting DR. Visual acuity and higher-order aberrations with wavefront-guided and wavefront-optimized laser in situ keratomileusis. Journal of Cataract and Refractive Surgery. 2010;36(3):437–41. doi: 10.1016/j.jcrs.2009.09.031. [DOI] [PubMed] [Google Scholar]
- 7.Yu J, Chen H, Wang F. Patient Satisfaction and Visual Symptoms After Wavefront-guided and Wavefront-optimized LASIK With the WaveLight Platform. Journal of Refractive Surgery. 2008;24(5):477–86. doi: 10.3928/1081597X-20080501-05. [DOI] [PubMed] [Google Scholar]
- 8.Wang L, Shoss BL, Weikert MP, Koch DD. Ocular Functional Optical Zone Changes Following Wavefront-Guided LASIK/PRK. Investigative Ophthalmology & Visual Science. 2010 Apr;51(13):4212. [Google Scholar]
- 9.Damgaard IB, Ang M, Mahmoud AM, Farook M, Roberts CJ, Mehta JS. Functional Optical Zone and Centration Following SMILE and LASIK: A Prospective, Randomized, Contralateral Eye Study. Journal of Refractive Surgery. 2019;35(4):230–7. doi: 10.3928/1081597X-20190313-01. [DOI] [PubMed] [Google Scholar]
- 10.Boxer Wachler BS, Huynh VN, El-Shiaty AF, Goldberg D. Evaluation of corneal functional optical zone after laser in situ keratomileusis. Journal of Cataract and Refractive Surgery. 2002;28(6):948–53. doi: 10.1016/s0886-3350(02)01322-6. [DOI] [PubMed] [Google Scholar]
- 11.El Danasoury AM, Holladay J, Waring GO, Pieger S, Bains HS. A Contralateral, Randomized Comparison of Optimized Prolate Ablation and Conventional LASIK for Myopia With the NIDEK Excimer Laser Platform. Journal of Refractive Surgery. 2012;28(7):453–61. doi: 10.3928/1081597X-20120621-01. [DOI] [PubMed] [Google Scholar]
- 12.Holladay JT, Janes JA. Topographic changes in corneal asphericity and effective optical zone after laser in situ keratomileusis. Journal of Cataract and Refractive Surgery. 2002;28(6):942–7. doi: 10.1016/s0886-3350(02)01324-x. [DOI] [PubMed] [Google Scholar]
- 13.Hou J, Wang Y, Lei Y, Zheng X. Comparison of effective optical zone after small-incision lenticule extraction and femtosecond laser–assisted laser in situ keratomileusis for myopia. Journal of Cataract and Refractive Surgery. 2018;44(10):1179–85. doi: 10.1016/j.jcrs.2018.06.046. [DOI] [PubMed] [Google Scholar]
- 14.Ding X, Fu D, Wang L, Zhou X, Yu Z. Functional Optical Zone and Visual Quality After Small-Incision Lenticule Extraction for High Myopic Astigmatism. Ophthalmology and Therapy. 2021;10(2):273–88. doi: 10.1007/s40123-021-00330-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tabernero J, Klyce SD, Sarver EJ, Artal P. Functional Optical Zone of the Cornea. Investigative Opthalmology & Visual Science. 2007;48(3):1053–60. doi: 10.1167/iovs.06-0867. [DOI] [PubMed] [Google Scholar]
- 16.Nepomuceno RL, Boxer Wachler BS, Scruggs R. Functional optical zone after myopic LASIK as a function of ablation diameter. Journal of Cataract and Refractive Surgery. 2005;31(2):379–84. doi: 10.1016/j.jcrs.2004.04.073. [DOI] [PubMed] [Google Scholar]
- 17.He L, Liu A, Manche EE. Wavefront-Guided Versus Wavefront-Optimized Laser in situ Keratomileusis for Patients With Myopia: A Prospective Randomized Contralateral Eye Study. American Journal of Ophthalmology. 2014;157(6):1170–8. doi: 10.1016/j.ajo.2014.02.037. [DOI] [PubMed] [Google Scholar]
- 18.Padmanabhan P, Mrochen M, Basuthkar S, Viswanathan D, Joseph R. Wavefront-guided versus wavefront-optimized laser in situ keratomileusis: Contralateral comparative study. Journal of Cataract and Refractive Surgery. 2008;34(3):389–97. doi: 10.1016/j.jcrs.2007.10.028. [DOI] [PubMed] [Google Scholar]
- 19.Sáles CS, Manche EE. One-Year Outcomes from a Prospective, Randomized, Eye-to-Eye Comparison of Wavefront-Guided and Wavefront-Optimized LASIK in Myopes. Ophthalmology. 2013;120(12):2396–402. doi: 10.1016/j.ophtha.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 20.Sales C, Manche E. One-year eye-to-eye comparison of wavefront-guided versus wavefront-optimized laser in situ keratomileusis in hyperopes. Clinical Ophthalmology. 2014;8:2229–38. doi: 10.2147/OPTH.S70145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khalifa MA, Alsahn MF, Shaheen MS, Pinero DP. Comparative analysis of the efficacy of astigmatic correction after wavefront- guided and wavefront-optimized LASIK in low and moderate myopic eyes. International Journal of Ophthalmology. 2017;10(2):285–292. doi: 10.18240/ijo.2017.02.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee W-S, Manche EE. Comparison of simulated keratometric changes following wavefront-guided and wavefront-optimized myopic laser-assisted in situ keratomileusis. Clinical Ophthalmology. 2018;12:613–9. doi: 10.2147/OPTH.S161387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roe JR, Manche EE. Prospective, Randomized, Contralateral Eye Comparison of Wavefront-Guided and Wavefront-Optimized Laser in Situ Keratomileusis. American Journal of Ophthalmology. 2019;207:175–83. doi: 10.1016/j.ajo.2019.05.026. [DOI] [PubMed] [Google Scholar]
- 24.Stonecipher K, Parrish J, Stonecipher M. Comparing wavefront-optimized, wavefront-guided and topography-guided laser vision correction. Current Opinion in Ophthalmology. 2018;29(4):277–85. doi: 10.1097/ICU.0000000000000495. [DOI] [PubMed] [Google Scholar]
- 25.Racine L, Wang L, Koch DD. Size of Corneal Topographic Effective Optical Zone: Comparison of Standard and Customized Myopic Laser In Situ Keratomileusis. American Journal of Ophthalmology. 2006;142(2):227–32. doi: 10.1016/j.ajo.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 26.Smolek MK, Klyce SD, Hovis JK. The Universal Standard Scale. Ophthalmology. 2002;109(2):361–9. doi: 10.1016/s0161-6420(01)00888-0. [DOI] [PubMed] [Google Scholar]
- 27.Partal AE, Manche EE. Diameters of topographic optical zone and programmed ablation zone for laser in situ keratomileusis for myopia. J Refract Surg. 2003;19(5):528–33. doi: 10.3928/1081-597X-20030901-07. [DOI] [PubMed] [Google Scholar]
- 28.Meidani A, Tzavara C. Comparison of efficacy, safety, and predictability of laser in situ keratomileusis using two laser suites. Clinical Ophthalmology. 2016;10:1639–46. doi: 10.2147/OPTH.S110626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hassan A, Massoud T, Nouby G, Fathlla A. Comparison of visual outcomes and higher order aberrations of wavefront-optimized and wavefront-guided myopic laser in-situ keratomileusis. The Egyptian Journal of Cataract and Refractive Surgery. 2017;23(1) [Google Scholar]
- 30.Mahmoud G, Massoud T, Fathlla A, Ahmed A. Evaluation of visual and refractive outcomes of wavefront optimized versus wavefront guided laser-assisted in situ keratomileusis in patients with myopia and myopic astigmatism. Journal of Current Medical Research and Practice. 2017;2(2):111–8. [Google Scholar]
- 31.Ghoneim A, Wasfy T, Abass S, Elbedewy H, Khater M, Khalifa M, et al. Comparative analysis of outcomes after wavefront-guided and wavefront-optimized laser in-situ keratomileusis in high myopic eyes. The Egyptian Journal of Cataract and Refractive Surgery. 2018;24(1) [Google Scholar]
- 32.Reed DS, Apsey D, Steigleman W, Townley J, Caldwell M. Retrospective Analysis of the Post-Operative Changes in Higher- Order Aberrations: A Comparison of the WaveLight EX500 to the VISX S4 Laser in Refractive Surgery. Military Medicine. 2017;182(11):e2061–e5. doi: 10.7205/MILMED-D-17-00159. [DOI] [PubMed] [Google Scholar]
- 33.Bottos KM, Leite MT, Aventura-Isidro M, Bernabe-Ko J, Wongpitoonpiya N, Ong-Camara NH, et al. Corneal asphericity and spherical aberration after refractive surgery. Journal of Cataract and Refractive Surgery. 2011;37(6):1109–15. doi: 10.1016/j.jcrs.2010.12.058. [DOI] [PubMed] [Google Scholar]
- 34.Molchan RP, Taylor KR, Panday VA, Caldwell MC, Reilly CD. Retrospective Analysis Comparing the Preoperative and Postoperative “Q” Values for 2 Different Lasers in Refractive Surgery. Cornea. 2015;34(11):1437–40. doi: 10.1097/ICO.0000000000000611. [DOI] [PubMed] [Google Scholar]