Figure 3.
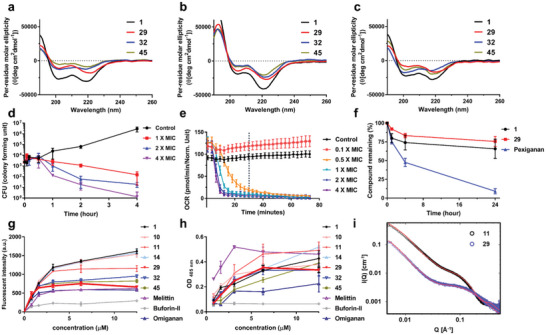
Conformational, biochemical, and mechanistic analysis of peptoids and AMPs. CD spectra of antimicrobial peptoids (50 µM) at 20 °C: a) in 5 mM Tris‐HCl buffer, b) in 5 mM lipid vesicles (POPE:POPG = 7:3 in molar ratio) in 10 mM Tris‐HCl buffer, c) in 5 mM lipid vesicles (POPC:cholesterol = 1:1 in molar ratio) in 10 mM Tris‐HCl buffer. d) Killing Kinetics. Time – killing study of E. coli ATCC 25922 challenged with various concentrations of 29. e) The real‐time oxygen consumption rate (OCR, expressed in picomoles of molecular oxygen per minute) of E. coli ATCC 25922 treated with various concentrations of 29. Dotted line indicates 30 min. f) Stability of peptoids (1 and 29) and pexiganan monitored after treatment with the human liver S9 fraction. g) Effect of antimicrobial peptides/peptoids on bacterial outer membrane tested with the NPN uptake assay in E. coli ATCC 25922. h) Effect of antimicrobial peptides/peptoids on bacterial inner membrane using an ONPG hydrolysis assay in E. coli ATCC 25922. i) SAXS data of peptoids 11 and 29 revealing distinct solution self‐assembled structures. SAXS data was collected in an aqueous environment at 5 mg mL−1 and plotted together with a best fit model (red line). Q is the scattering vector as defined in the experimental section. The y‐axis indicates scattering intensity (I).
