SUMMARY
Effective tuberculosis (TB) prevention and care for migrants requires population health-based approaches that treat the relationship between migration and health as a progressive, interactive process influenced by many variables and addressed as far upstream in the process as possible. By including capacity building in source countries, pre-migration medical screening has the potential to become an integral component of public health promotion, as well as infection and disease prevention, in migrant-receiving nations, while simultaneously increasing capabilities in countries of origin. This article describes the collaborative experiences of five countries (Australia, Canada, New Zealand, United Kingdom and the United States of America, members of the Immigration and Refugee Health Working Group [IRHWG]), with similar pre-migration screening programmes for TB that are mandated. Qualitative examples of capacity building through IRHWG programmes are provided. Combined, the IRHWG member countries screen approximately 2 million persons overseas every year. Large-scale pre-entry screening programmes undertaken by IRHWG countries require building additional capacity for health care providers, radiology facilities and laboratories. This has resulted in significant improvements in laboratory and treatment capacity, providing availability of these facilities for national public health programmes. As long as global health disparities and disease prevalence differentials exist, national public health programmes and policies in migrant-receiving nations will continue to be challenged to manage the diseases prevalent in these migrating populations. National TB programmes and regulatory systems alone will not be able to achieve TB elimination. The management of health issues resulting from population mobility will require integration of national and global health initiatives which, as demonstrated here, can be supported through the capacity-building endeavours of pre-migration screening programmes.
Keywords: capacity building, intergovernmental, migration, population health
RESUME
Une prévention et une prise en charge efficaces de la tuberculose (TB) chez les migrants requièrent des approches basées sur la santé des populations qui tiennent compte de la relation entre migration et santé comme un processus progressif, interactif, influencé par de nombreuses variables, et la question doit être affrontée le plus en amont possible de ce processus. En incluant le renforcement des capacités dans les pays sources, le dépistagemédical préalable à la migration a le potentiel de devenir un élément intégral de promotion de la santé publique ainsi que de la prévention de l’infection et de la maladie dans les nations d’accueil des migrants, tout en augmentant les capacités dans les pays d’origine. Cet article décrit les expériences de collaboration de cinq pays (Australie, Canada, Nouvelle-Zélande, Royaume-Uni et Etats-Unis d’Amérique, membres du groupe Immigration and Refugee Health Working Group [IRHWG]) avec des programmes de dépistage de la TB préalables à la migration qui sont imposés. L’article fournit des exemples qualitatifs de renforcement des capacit és à travers les programmes de l’IRHWG. Ensemble, les pays membres d’IRHWG dépistent plus de 2 millions de personnes dans le monde chaque année. Les programmes de dépistage à grande échelle, préalables à l’entrée, entrepris par les pays de l’IRHWG demandent davantage de renforcement des capacités pour les prestataires de soins, les centres de radiologie et les laboratoires. Ceci a abouti à des améliorations significatives des capacités de laboratoire et de traitement, qui profitent donc également aux programmes nationaux de santé publique. Aussi longtemps qu’existent les disparités mondiales en matière de santé et les différences en termes de prévalence des maladies, les programmes nationaux de santé publique et les politiques des nations recevant les migrants seront confrontées au défi de la prise en charge des maladies prévalentes dans ces populations migrantes. Les programmes nationaux tuberculose et les systèmes de règlementation seuls ne seront pas capables d’aboutir à l’élimination de la TB. La gestion des questions de santé résultant de la mobilité des populations exigera une intégration des initiatives de santé nationales et mondiales, qui, comme cela a été démontré ici, peut être soutenue grâce à des initiatives de renforcement des capacités des programmes de dépistage avant la migration.
RESUMEN
La eficacia en la prevención y la atención de la tuberculosis (TB) en los migrantes exige estrategias poblacionales de salud que tengan en cuenta la correlación que existe entre la migración y la salud como un proceso progresivo e interactivo que depende de muchas variables y que abordan estos factores lo más cerca posible de su origen. El examen médico de detección sistemática antes de la migración, al contribuir a desarrollar medios de acción en los países de origen, se puede convertir en un componente integral de la promoción de salud pública y una iniciativa de prevención de la infección y la enfermedad tuberculosa en los países que acogen a los migrantes, que a su vez fortalecen la capacidad de los países origen. En el presente artículo se describen las experiencias de colaboración de cinco países (Australia, Canadá, Nueva Zelanda, el Reino Unido y los Estados Unidos de América) miembros del grupo de trabajo IRHWG (por Immigration and Refugee Health Working Group), que cuentan con programas equivalentes de detección sistemática obligatoria antes de la migración. Se aportan ejemplos cualitativos de creación de capacidades por conducto de los programas del IRHWG. En conjunto, los países miembros del IRHWG examinan en el extranjero a más de 2 millones de personas por año. Los programas de detección sistemática en gran escala antes de la entrada que han emprendido estos países exigen la creación adicional de aptitudes en los trabajadores de atención de salud y el aumento de la capacidad de instalaciones radiográficas y de laboratorio. El programa ha dado lugar a progresos considerables en materia de medios de laboratorio y de tratamiento, al poner estas facilidades a la disposición de los programas nacionales de salud pública. En la medida en que perduren las disparidades mundiales con respecto a la salud y la prevalencia de enfermedades, los programas y las políticas nacionales de salud pública de los países de acogida seguirán afrontando las dificultades de prestar atención a las enfermedades predominantes en estas poblaciones migrantes. Los programas nacionales contra la TB y las autoridades normativas no lograrán por sí solos la eliminación de la TB. La gestión de los problemas de salud que surgen con la movilidad de las poblaciones exigirá una integración de las iniciativas nacionales y mundiales relacionadas con la salud, que como se demuestra en este análisis, se pueden fortalecer mediante las actividades de los programas de detección sistemática anteriores a la migración.
THE IMMIGRATION and Refugee Health Working Group (IRHWG) is a partnership of member states that gathers government officials from Australia, Canada, New Zealand, the United Kingdom and the United States of America on a regular basis for information exchange, agreement and cooperation, with the common goal of optimising international best practices for the screening and treatment of prospective migrants and effective management of communicable health risks, and the overriding priority of protecting public health. The group is not a legally constituted body, but rather a consultative forum that seeks to enhance the health security of migrants and receiving countries, the health services provided to migrants, and tuberculosis (TB) prevention and care globally. The purpose of this article is to describe the screening programmes, provide qualitative examples of capacity building that have occurred through these requirements and highlight how this capacity can be used to benefit broader management efforts.
All five countries have pre-migration screening programmes for TB that are mandated through legislation. These programmes have been in place in some countries for many years: Australia and New Zealand from 1901 and 1899, respectively, and Canada since 1869.1 In the United Kingdom, pre-migration screening replaced port-of-entry screening in 2014, following a successful pilot in 15 high TB incidence countries.2,3
These pre-migration TB screening programmes are administered by various agencies in IRHWG countries and include the Department of Immigration and Border Protection (DIBP) in Australia; Immigration, Refugees and Citizenship Canada; Immigration New Zealand (INZ); UK Home Office and Public Health England; and, in the United States, the Centers for Disease Control and Prevention (CDC). The purpose of these programmes is similar—to prevent the importation of certain communicable diseases. All five countries screen for infectious TB.4–8 Australia and the United States also have a requirement to screen for latent tuberculous infection (LTBI), in which children aged 2–11 years in Australia or 2–14 years in the United States undergo a tuberculin skin test (TST) or an interferon-gamma release assay (IGRA) if they are examined in a country with an elevated rate of TB (⩾40 per 100 000 for Australia, ⩾20 per 100 000 for the United States); treatment for LTBI is provided after arrival in the receiving country. For Australia, Canada and New Zealand, there is also a legislative requirement to avoid excessive health system costs.
The IRHWG partners together annually screen approximately 2 million immigrants (applicants for permanent entry), refugees and long-term visitors (individuals planning temporary stays for ⩾6 months, such as international workers and international students) overseas prior to travel. While the source countries vary among the five partners, the dominant caseloads come from Asia, with India, China, the Philippines and Viet Nam frequently in the top five.9–11 These countries are all classified by the World Health Organization (WHO) as high TB burden countries.12
ADMINISTRATION OF PRE-MIGRATION HEALTH ASSESSMENT PROGRAMMES
Examinations of applicants bound for the five countries are performed through similar and consistent processes by ‘panel physicians’: licensed physicians in the countries of origin that have agreements with the government departments of the country of destination to undertake this activity. These agreements may be formal and written (United States), letter only (Australia, Canada and New Zealand) or a contract (United Kingdom, for whom the physicians are also listed in legislation).
Panel clinics, often shared between these partner countries, are numerous, with 800 sites in over 170 countries. Four of the five countries provide panel physicians with their individual Technical Instructions, which stipulate how the examination should be performed.13–16 Canada requires its panel physicians to adopt standards set by the national tuberculosis programmes (NTPs) in each country, augmented with WHO TB treatment recommendations and the latest Canadian standards (G Giovinazzo, personal communication). Historically, each country undertakes monitoring and oversight activities of its networks and provides specific education and training of panel physicians. Collaborative efforts by the five countries through shared expertise have recently developed a non-binding set of common specifications, providing a standard approach to TB screening and management for panel physicians.17
Not all migrants are screened for TB. Policies vary among the different countries, balancing the need to protect public health and the practicalities of screening all individuals considered to have a high TB risk. Other considerations in developing screening policies include the duration and purpose of the visit and concerns that the cost of screening may act as a barrier to those seeking entry.
Australia, Canada, New Zealand and the United States screen all refugees relocating to their countries and all permanent migrants, irrespective of TB incidence in the country of origin. Australia, Canada and New Zealand also undertake pre-migration screening for those coming for temporary stays of ⩾6 months from countries with a WHO-estimated TB incidence of >40/100 000. The United Kingdom screens all refugees relocating to its country, all permanent migrants and those coming for temporary stays of ⩾6 months from countries with a WHO-estimated TB incidence12 of.>40/100 000 (Table 1).
Table 1.
Pre-migration screening programmes of the Immigration and Refugee Health Working Group countries* and TB screening results for 2014
| Country | Population screened overseas† | Minimum TB rate for countries subject to screening /100 000 | Examinations‡ n |
TB cases n |
TB rate screened /100 000 |
|---|---|---|---|---|---|
| Australia | Long-term visitors§ | Any¶ | 530801 | 425 | 80 |
| Canada | Long-term visitors§ | Any¶ | 304314 | 593 | 194 |
| New Zealand | Long-term visitors§ | Any¶ | Estimated 120 000 | Estimated 50 | 41 |
| United Kingdom | Long-term visitors§ | 40 | 233351 | 369 | 159 |
| United States | Immigrants and refugees‡ | Any | 631 100 | 1450 | 230 |
Australia, Canada, New Zealand, United Kingdom and the United States.
Does not include persons who apply for immigration domestically.
Number of examinations instead of number of individual persons screened, as some may have more than one examination if they fail to travel before the expiry of examination validity.
Permanent immigrants, refugees and temporary workers and students who will be in the country for ⩾6 months.
Any rate for permanent immigrants and refugees; 40/100 000 for temporary workers and students who will be in the country for ⩾6 months. TB = tuberculosis.
All five countries now have TB screening requirements that include a culture-based algorithm for TB disease screening. If applicants have TB symptoms or signs, or if the chest X-ray (CXR) has indications consistent with TB disease, the Technical Instructions require mycobacterial cultures and drug susceptibility testing (DST).13–17 In addition, for some of the destination countries that mandate treatment, these cases are required to be treated according to American Thoracic Society (ATS)/CDC/Infectious Diseases Society of America (IDSA) treatment guidelines,13,18 with all doses of treatment delivered as directly observed therapy (DOT), whereas others require treatment according to in-country, WHO or their respective IRHWG country’s standards (Canada, unpublished requirements).14–17
EFFECTIVENESS OF PRE-MIGRATION SCREENING IN REDUCING IMPORTED TUBERCULOSIS CASES
Diagnostic rates among countries vary. This is assumed to be due to different cohorts migrating, although further research is required to verify this. However, all identify large numbers of cases of TB disease through the pre-migration screening process, preventing diagnoses of TB disease after arrival and assisting in TB prevention and care.19–24 In 2014, US panel physicians conducted examinations in 631 100 migrants. Of these, 1450 were diagnosed with TB (rate 230/100 000), 1135 had positive culture and 802 of those with positive cultures had negative sputum smears (unpublished CDC data). In 2014, the yield for the United Kingdom was 159/100 000 (unpublished UK data), while Australia screened 530 801 migrants and diagnosed TB at a rate of 80/100 000 (unpublished Australia data). Canada estimated that its rate of detection was 194/100 000, while New Zealand estimated that panel physicians performed 120 000 examinations (Table 1).
The effectiveness of pre-migration screening has also been demonstrated with respect to the detection of drug-resistant TB, which would not be detected in the absence of rigorous screening programmes relying on culture and DST. In 2014, the US screening programme led to the diagnosis of 44 migrants with multidrug-resistant TB (MDR-TB) and one with extensively drug-resistant TB (XDR-TB; CDC, unpublished data, Table 1). The UK screening programme also detected several drug-resistant cases: between 2007 and 2015, about 1.7% of isolates were MDR-TB, 3.4% were polyresistant to first-line drugs and about 8.6% were isoniazid-monoresistant.22
From 2007 to 2013, CDC implemented new Technical Instructions in the United States requiring culture and DOT; these requirements remain in place.13 This strategy resulted in additional cases of TB being diagnosed overseas and coincided with a reduction in US TB cases diagnosed within the first year after arrival.19,20 Gains in overseas diagnoses coincided with an almost equivalent drop in domestic TB cases diagnosed in migrants within 1 year of arrival in the United States.20 In the United Kingdom, the number of prevalent pulmonary TB cases (notified in the United Kingdom within 1 year of entry) has decreased dramatically with increasing detection rates overseas.10,22 In Australia, previous research estimates have suggested that, without pre-migration screening in place, the incidence rate in Australia would be more than 30% higher than it currently is.21 In 2014, the number of active pulmonary TB cases detected in Canada using migration screening was more than 570. If these clients had entered Canada without being screened, it would have led to an increase of at least 40% in the number of active pulmonary TB cases in Canada (unpublished data, Public Health Agency of Canada). The effectiveness of these pre-migration screening programmes was significantly enhanced through the capacity-building endeavours outlined below.
CAPACITY BUILDING
To deliver large-scale pre-entry screening programmes requires building additional capacity for panel clinics, radiology facilities and laboratories. This may be accomplished using three specific processes dependent on current infrastructure or capacity in countries of origin. The first was the implementation and strengthening of pre-migration programmes by building on existing infrastructure. The second was to leverage specifically targeted priorities to develop programmes in countries of origin as part of a broader aid strategy or to deliver completely new infrastructure to support the sustainability of the screening programmes. The third was to build partnerships in-country and engage in strengthening NTPs.
These capacity-building approaches have resulted in numerous improvements in laboratory (Figure) and treatment capabilities, especially as many countries lacked adequate mycobacterial culture capacity, DST capacity (by either molecular or phenotypic testing), drug availability or DOT infrastructure. The availability of these laboratory and treatment facilities for NTPs, and broader engagement with private-sector providers, has sub-stantially increased the capacity for TB management in many countries.
Figure.
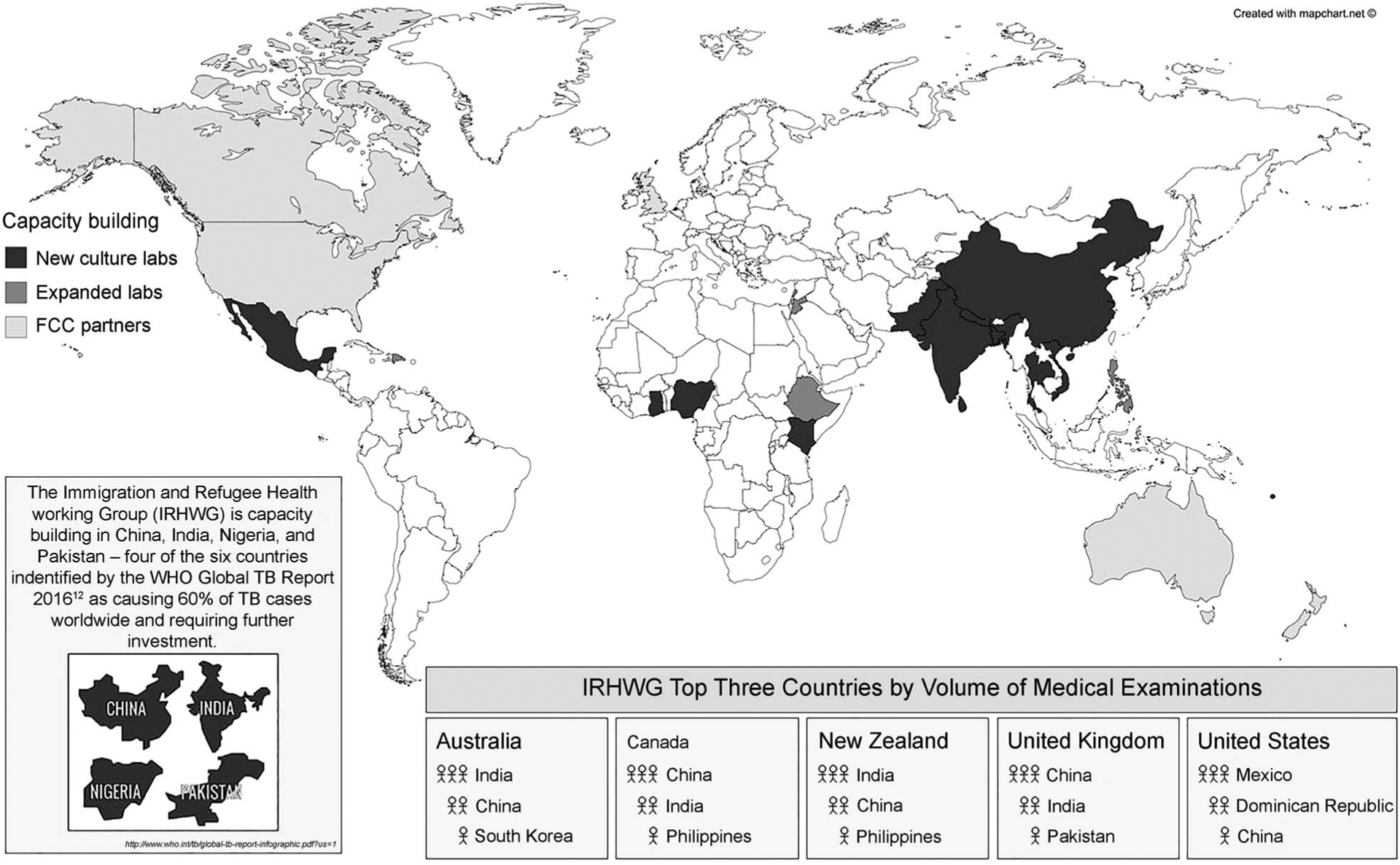
Expanded and new laboratory capability developed through pre-migration TB screening for Immigration and Refugee Health Working Group countries.* * Australia, Canada, New Zealand, United Kingdom, and United States. TB=tuberculosis; WHO=World Health Organization; FCC = Federal Communications Commission.
Increases in laboratory capacity
As shown in Table 2, new laboratories with TB (liquid) culture capacity have been developed in many countries and laboratories in several countries have been greatly expanded. In addition to culture, many also perform first-line DST and some perform second-line DST. Many laboratories also now have access to molecular tests, including the GenoType® MTBDRplus assay (Hain Lifescience, Nehren, Germany) and Xpert® MTB/RIF assay (Cepheid, Sunnyvale, CA, USA).
Table 2.
Capacity building through pre-migration TB screening for Immigrant and Refugee Health Working Group countries*
| Type of capacity building | Countries |
|---|---|
| New culture laboratories since 2007 | Bangladesh, China, Ghana, India, Kenya, Mexico, Nepal, Nigeria, Pakistan, Sri Lanka, Thailand, Vanuatu, Viet Nam, the Philippines |
| Second-line DST developed since 2007 | China, Kenya, Nepal, Thailand, Viet Nam, Nigeria |
| Specimen testing, training or treatment for local TB institutions | China, Kenya, Mexico, Nigeria, South Sudan |
| Public-private partnerships (panel physicians providing training and education for local TB providers or assisting with importing second-line drugs) | Dominican Republic, Ethiopia, Viet Nam |
| Engagement with global TB community—IOM Awards from Stop TB Partnership’s TB REACH | Ethiopia, Thailand |
| Leveraging refugee programmes for broader refugee source population efforts | Nepal, Kenya |
Australia, Canada, New Zealand, United Kingdom and the United States.
TB = tuberculosis; DST = drug susceptibility testing; IOM = International Organization for Migration.
Greater individualised treatment and DOT
TB treatment based on pre-entry screening is carried out in the countries of origin, and all countries with designated screening sites must have at least one location that provides treatment according to international standards in which every dose is delivered as DOT. For TB cases that may be more difficult to treat, panel physicians for the United States and Australia have access to clinical experts in destination countries. Access to external TB experts increases the level of knowledge among physicians managing TB cases in sender countries.
Training and education of panel site personnel
All receiving countries help train medical and administration staff of panel clinics in TB, and contribute to an annual panel physicians’ training summit carried out in collaboration with the International Panel Physicians Association (IPPA; El Paso, TX, USA), a non-governmental organisation serving as a professional association for panel physicians. Beginning from 2013, these summits have had approximately 300 panel physician and staff and consular staff attendees yearly. In addition to learning from each of the IRHWG countries, panel physicians and their staff learn from international TB experts through lectures and interactive workshops. IRHWG also supports e-learning training activities, including webinars conducted by the CDC since 2010 and a joint IRWHG webinar on radiology in 2013. In addition, the CDC and the DIBP have been carrying out smaller regional training events since 2008 and 2010, respectively, which are each attended by 30–50 panel physicians.
In addition to direct teaching activities, capacity building also occurs through broader processes. These include the provision of tools for patient education (e.g., CDC posters on sputum collection, radiography books for staff education), assistance in developing local operating procedures (especially for sputum collection) or, more directly, through quality assurance visits by IRHWG staff to approximately 50–60 countries per year or through IPPA peer-to-peer site visits, at which panel physicians and staff receive lessons specific to their local environment and network with other panel physician colleagues. As a group, the IRHWG countries conduct site visits each year to large- and small-volume panel sites in the Americas, Europe, Middle East, Africa and Asia. Since 2014, IPPA has been conducting peer-to-peer site visits to three countries per year that IRWHG was not able to visit.
Linkages between screening programmes and in-country TB providers
In addition to developing laboratory and treatment infrastructure, a key element specific to the CDC programme is to build linkages between screening programmes and in-country TB providers. Through these linkages, panel physicians have relationships with other in-country TB providers, such that programmes for IRHWG-bound populations would also benefit in-country management efforts. Australia and New Zealand have more recently targeted similar, jointly managed strategies within the South Pacific region. While IRWHG lacks data on the number of specimens or number of non-migrating persons who receive treatment through a panel physician-local institution linkage, there are several examples of these types of linkages.
US panel physicians have achieved some notable partnership agreements. In the Americas, Consultorios de Visa (CDV), a panel site in the Dominican Republic, established a public-private partnership with the NTP whereby CDV provides training to NTP staff on radiology interpretation and mentor-ship for NTP efforts in two prisons. Moreover, the laboratory used by CDV, Laboratorio Referencia, provides training to NTP staff as well. In Mexico, two panel physician sites in Ciudad Juarez, Clinica Medica Internacional and Servicios Medicos de la Frontera, collaborate on a laboratory that also performs sputum testing for other TB programmes serving the binational population along the US-Mexico border. Laboratories supporting IRHWG programmes in Chengdu and Shenyang, China, also perform testing for the community.
In Africa, the International Organization for Migration (IOM), which serves as the screening provider for the majority of the refugees resettled by IRHWG countries, has collaborated with the Kenya NTP through the establishment of a DOT site in Eastleigh, a Nairobi neighbourhood; the IOM laboratory in Nairobi also processes specimens for the NTP. This IOM laboratory is a key service provider assisting the Nigerian NTP in Abuja by providing second-line DST for cases identified as rifampicin-resistant in the NTP laboratory using Xpert, as well as the principal laboratory supporting the diagnosis and treatment of MDR-TB cases in refugees who migrate from Somalia to the Dadaab refugee camp.25 IOM has also worked to provide assistance with sputum smear testing in South Sudan. In addition to its work in Africa, the IOM has been identified as lead coordinator in assisting NTPs to roll out screening programmes for migrant and refugee groups in Lebanon and Jordan.
In Asia, for several years, IRHWG countries have been receiving Bhutanese refugees located in several camps in the eastern part of Nepal, where the NTP has limited infrastructure. To ensure appropriate diagnosis and treatment of TB among the resettling population, the United States and Canada have provided funding for IOM to partner with the Association of Medical Doctors, a non-governmental organisation in the region, to provide access to culture, DST and DOT for the camp population.
New Zealand and Australia have targeted the current TB ‘hot spots’ in the South Pacific and South-East Asian regions, principally through aid programmes. One example from INZ in the South Pacific is in Vanuatu, where TB diagnosis and screening have been strengthened. Furthermore, the introduction of electronic reporting of CXR results by radiologists elsewhere in the region has resulted in building knowledge and capacity in local clinicians who have not had access to this expertise previously (Table 3).
Table 3.
Best practice examples of linkages between panel physicians conducting medical examinations for Immigration and Refugee Health Working Group countries* and in-country TB programmes
| Type of linkage | |
|---|---|
| Public-private partnership | Consultorios de Visa, a panel physician site in Santo Domingo, Dominican Republic, has a public-private partnership with the Dominican Republic NTP. Through this partnership, the NTP provides treatment to applicants diagnosed with TB disease, while Consultorios de Visa provides training to NTP staff on digital radiology, the tuberculin skin test and the interferon-gamma release assay |
| Laboratory services | Among the laboratories that support these programmes, some also support in-country TB programmes. In Kenya, the IOM has developed a large, fully equipped TB laboratory service. Good collaborative relationships have been developed with the NTP in Kenya, thereby enabling the NTP to also benefit from this laboratory service. Similarly, a recently developed IOM TB laboratory service in Abuja, Nigeria, can support the Nigerian NTP through drug susceptibility testing for samples identified as rifampicin-resistant through the Cepheid Xpert® MTB/RIF assay. Laboratorios Medicos Especializados, a laboratory in Cuidad Juarez, Mexico, performs testing for the immigrants, and also for a local non-governmental organisation that provides treatment to TB cases, along the US-Mexico border |
| Treatment | In Eastleigh, Nairobi, Kenya, IOM and the Kenyan NTP collaborated to establish a new site for DOT, and IOM also acts as the principal provider to manage MDR-TB cases who migrated from Somalia to the Dadaab refugee camp |
Australia, Canada, New Zealand, the United Kingdom and the United States.
TB=tuberculosis; NTP=national tuberculosis programme; IOM=International Organization for Migration; DOT=directly observed therapy; MDR-TB = multidrug-resistant TB.
DISCUSSION
This analysis helps to demonstrate that, because the number of panel physicians is large in many high TB incidence source countries, IRHWG countries are uniquely positioned to ensure that their investments in screening programmes also contribute to local prevention and treatment efforts through the development of relationships with TB controllers by sharing laboratory capacity and co-managing TB cases where DOT capacity is scarce.11 This means that a strategy to develop infrastructure in IRHWG screening programmes also has the potential to have a domestic impact in each IRHWG country, as well as contributing to global TB efforts.26 Because many of these examples are in countries with both a high TB incidence and low levels of TB infrastructure, as we have seen, this collaborative effort has catalysed laboratory and treatment infrastructure or training and education activities that may not otherwise have been possible. IRHWG programme efficiency and effectiveness could be further enhanced by pooling resources such as laboratory, radiology and examining physicians. The high standards of radiological and laboratory diagnosis required by screening countries are often in short supply in high-incidence regions. More robust TB services in high TB incidence regions support TB prevention in migrants from those areas and in future host countries. Based on the evidence, it is recommended that panel physicians build relationships with the NTPs in their countries and explore opportunities for further collaboration to improve TB diagnosis and treatment in source populations.
In a connected global environment, borders are no longer an ‘edge’ but a ‘continuum’ for migrant health, which begins at the host country and continues to after arrival in the destination country, with a series of partners and agencies in both countries of destination and origin working collaboratively, including TB screening programmes. Preventing importation of TB into low TB incidence countries requires an ‘enlightened self-interest approach’ of capacity building in the countries of origin.27 Requiring rigorous overseas TB screening programmes for migration and refugee resettlement results in the development of laboratory and treatment capacity.21
In recent decades, the number of international migrants has increased, and is estimated at 244 million globally, about one in every 30 of the world’s inhabitants.28 While most of these individuals migrate within their world region, a substantial number come to low TB incidence countries. Addressing TB in migrating populations is key to global TB elimination efforts under the WHO’s post-2015 End TB global strategy.29 Migrant populations face a spectrum of determinants that make them particularly vulnerable to disease, and migration itself is a social determinant of health that may increase TB-related morbidity and mortality among mobile populations.30
International migration, a social phenomenon caused by various push and pull factors, including poverty, conflict, and, in some countries, an increasingly ageing workforce, influences the health of individuals and populations.31–33 These migrant networks, no longer a one-way trajectory, increase ties between global and local communities,31,33 where migration acts as a bridge across borders for people with different health profiles that inevitably have an effect on disease rates, health care access and health-seeking behaviours in the receiving countries.31–35
In lower TB incidence receiving countries, the health of migrants contributes to TB epidemiology through the importation, potential transmission and progression of disease. International migration reduces the effects of distance and results in rapid links that have implications for preventive care.36 This concept of ‘transnational neighbourhoods’ with frequent border crossings that span hundreds or thousands of kilometres is therefore more important in planning TB prevention and care than the historical nature of dealing with this at a national level as if there is only a single border crossing point.36,37
The primary focus of panel physicians is to conduct medical examinations and comply with the requirements of the IRHWG countries. In doing so, there is a risk that the physicians could operate somewhat independently of the health care systems of their countries. If this were to occur, the increases in TB capacity would only benefit the populations that are leaving the country. While that is still a benefit, IRHWG countries identify that with the changing patterns of migration, there is significant benefit in also preventing and treating TB more broadly in countries of origin. Hence, IRHWG countries are committed to encouraging panel physicians to engage with their ministries of health, NTPs and other TB providers to build relationships, share epidemiologic data, share expertise and allow capacity building for migrant screening programmes to benefit more than IRHWG-bound populations.
Effective addressing of migrant TB requires health-based population approaches that consider the relationship between migration and health as a progressive, interactive process influenced by temporal and local variables,37 and as far upstream in the process as possible. For receiving countries, the primary intent of screening pre-migration is to achieve this ‘protection’ as early in the process as possible, with linkage to local treatment and surveillance programmes. This creates the potential to assist the individual, as well as the country of origin, through partnerships and infrastructure that address these health needs.
This response can be described as ‘global public health good’, defined as an intervention and service whose benefits cross borders and profit source communities.38 The capacity-building endeavours described above that increase services at origin for all, as well as facilitating integration into the health systems at the destination, are examples of global public health benefit. Pre-migration screening, in this context, has the potential to become an integral component of public health promotion and disease prevention in migrant-receiving countries,23,35,37 while simultaneously delivering capability in the country of origin.
It has been reported that migrants screened for TB disease before entry pose a negligible risk in terms of onward transmission in their receiving country,23,32 while their individual risk remains increased. It has also been noted that policies to protect the health of migrants as well as public health will be most effective if they address the continuum of the migratory process, including pre-departure, travel, arrival at destination and return, with health intervention opportunities existing at each stage.31
Pre-migration-phase TB screening programmes from Australia, Canada, New Zealand, the United States and the United Kingdom consistently show the effectiveness of early diagnosis and TB management in migrants.20,39–42 These collaborative efforts could yield sizeable gains in TB mitigation for migrant- and refugee-receiving countries11 and, through the capacity and linkages developed overseas, also provide sizeable contributions to source-country TB programmes.
Decades of implementing passive TB case finding methods have demonstrated the limitations of comprehensive and early detection of TB in making significant improvements in TB outcomes. Implementation of WHO strategies on TB screening released in 201343 can substantially reduce TB in high-incidence countries, but due to insufficient funding global implementation is far from complete. Many countries have poor infrastructure, inadequate or outdated equipment with poor biosafety measures, and scarce human and financial resources, leading to delays in diagnosis and treatment.44,45 Support for these programmes has many challenges and requires investment in leadership development.32 Systematic screening for active TB could help address these limitations,37 and screening migrants in this respect plays a crucial role, including capacity building.
Evidence suggests that domestic returns and more cost-effective outcomes could be obtained through interventions focused on disease-containment efforts in source countries alongside pre-entry screening programmes.27 This broader view would enhance global collaboration efforts to eliminate TB.41
The long-term goal in reducing migration-related introduction of TB from high- to low-incidence countries means diminishing the prevalence of the disease in those high-incidence source locations.11 As argued, overseas TB screening programmes for migration and refugee resettlement contribute to this goal through the development of laboratory and treatment capacity.
The global TB epidemic can be improved by taking advantage of the motivation that drives more than one billion mobile individuals to seek a better future using pre-migration screening to prevent infectious TB from crossing borders and using screening programmes as investments in sender countries.46 Thus, while individual country efforts in managing TB screening programmes are invaluable for reducing importation of TB, they should also be leveraged to assist with efforts in the source countries.47 Improved linkages between panel physician activities and NTPs in their countries benefit migrants and others in the source populations.
CONCLUDING REMARKS
Health policy making in the context of migration has generally been viewed either in terms of its ‘threats’ to public health or from a rights-based approach that focuses on health hazards faced by individual migrants and the associated service challenges.31,48 The convergence of more rigorous international protocols and growing capacity among panel physicians presents a unique opportunity to contribute to meeting elimination targets. Enhanced, synergised screening protocols across IRHWG countries enable panel physicians to meet the public health standards of receiving countries while maximising programme effectiveness through capacity building and delivering the highest standards of care in host countries. This requires all participants and stakeholders to play proactive, strategic and systematic roles to link the management of TB to broader capacity-building needs.
The net result is an ongoing globalisation of health influences and indicators currently relevant at both national and global levels. As long as global health disparities and prevalence differentials exist, national health programmes and policies in migrant-receiving nations will continue to be challenged to manage the diseases prevalent in these migrating populations. To be effective, the management of health issues resulting from population mobility will require the integration of national and global health initiatives which, as demonstrated here, can be supported through the capacity-building endeavours of pre-migration screening programmes.
Footnotes
Disclaimer: The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the Centers for Disease Control and Prevention or other government entities.
Conflicts of interest: none declared.
References
- 1.Dara M, Gushulak BD, Posey DL, Zellweger JP, Migliori GB. The history and evolution of immigration medical screening for tuberculosis. Expert Rev Anti Infect Ther 2013; 11: 137–146. [DOI] [PubMed] [Google Scholar]
- 2.Severi E, Maguire H, Ihekweazu C, Bickler G, Abubakar I. Outcomes analysis of new entrant screening for active tuberculosis in Heathrow and Gatwick airports, United Kingdom 2009/2010. BMC Infect Dis 2016; 16: 178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muzyamba M, Aldridge R, Ekeke N, Abubakar I, Zenner D. UK pre-entry tuberculosis screening brief report 2013. London, UK: Gov.UK, 2013. [Google Scholar]
- 4.United States Government. Medical Examination of Aliens—Revisions to Medical Screening Process. Washington DC, USA: US Government, 2016. Final Rule Federal Register 2016; 81: 4191–4206. https://www.gpo.gov/fdsys/pkg/FR-2016-01-26/pdf/2016-01418.pdf Accessed April 2017. [PubMed] [Google Scholar]
- 5.Gov.UK. Tuberculosis: pre-entry screening in the UK. London, UK: Gov.UK, 2017. https://www.gov.uk/government/publications/tuberculosis-pre-entry-screening-in-the-uk. Accessed April 2017. [Google Scholar]
- 6.Government of Canada. Immigration and Refugee Protection Act (SC 2001, c. 27). Ottawa, ON, Canada: Government of Canada, 2001. http://laws.justice.gc.ca/eng/acts/i-2.5/. Accessed April 2017. [Google Scholar]
- 7.Immigration New Zealand. New Zealand Operations Manual. Wellington, New Zealand: INZ, 2017. http://onlineservices.immigration.govt.nz/opsmanual/?_ga=1.193272997.983730666.1441326118. Accessed April 2017. [Google Scholar]
- 8.Government of Australia. Australia Migration Regulations 1994—Schedule 4. Canberra, ACT, Australia: Government of Australia, 1994. http://www.austlii.edu.au/au/legis/cth/consol_reg/mr1994227/sch4.html. Accessed April 2017. [Google Scholar]
- 9.United States Department of Homeland Security. Yearbook of Immigration Statistics: 2014. Washington, DC, USA: US Department of Homeland Security, Office of Immigration Statistics, 2016. [Google Scholar]
- 10.Public Health England. Tuberculosis in England 2016 Report. London, UK: PHE, 2015. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/492431/TB_Annual_Report_v2.6_07012016.pdf Accessed April 2017. [Google Scholar]
- 11.White ZA, Painter J, Douglas P, et al. Immigrant arrival and TB trends among large immigrant and refugee receiving countries 2005–2009. Tuberculosis Research and Treatment 2017, Article ID 8567893. https://www.hindawi.com/journals/trt/2017/8567893/ Accessed April 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization. Global tuberculosis report, 2016. WHO/HTM/TB/2016.13. Geneva, Switzerland: WHO, 2016. http://www.who.int/tb/publications/global_report/en/. Accessed April 2017. [Google Scholar]
- 13.Centers for Disease Control and Preventions. Technical instructions for panel physicians. Atlanta, GA, USA: CDC, 2017. http://www.cdc.gov/immigrantrefugeehealth/exams/ti/panel/technical-instructions-panel-physicians.html. Accessed April 2017. [Google Scholar]
- 14.Australian Government, Department of Immigration and Border Protection. Australia’s Panel Physician Instructions. Canberra, ACT, Australia: Australian Government, 2016. https://www.border.gov.au/Panelphysician-s/Documents/panel-member-instructions.pdf Accessed April 2017. [Google Scholar]
- 15.Gov.UK. Tuberculosis technical instructions. London, UK: Gov. UK, 2013. https://www.gov.uk/government/publications/uk-tuberculosis-technical-instructions. Accessed April 2017. [Google Scholar]
- 16.Immigration New Zealand. New Zealand panel physician instructions. Wellington, New Zealand: INZ, 2017. https://www.immigration.govt.nz/assist-migrants-and-students/other-industry-partners/medical-professionals/panel-physician-instructions. Accessed April 2017. [Google Scholar]
- 17.Immigration and Refugee Health Working Group. Considerations for technical specifications on tuberculosis screening and treatment: IRHWG internal document. Geneva, Switzerland: International Organization for Migration, 2016. [Google Scholar]
- 18.Nahid P, Dorman SE, Alipanah N, et al. Official American Thoracic Society/Centers for Disease Control and Prevention/ Infectious Diseases Society of America Clinical Practice Guidelines: treatment of drug-susceptible tuberculosis. Clin Infect Dis 2016; 63: e147–e195. http://cid.oxfordjournals.org/content/63/7/e147. Accessed April 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Posey DL, Naughton MP, Willacy EA, et al. Implementation of new TB screening requirements for U.S.-bound immigrants and refugees, 2007–2014. MMWR Morb Mortal Wkly Rep 2014; 63: 234–236. [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Y, Posey DL, Cetron MS, Painter JA. Effect of a culture-based screening algorithm on tuberculosis incidence in immigrants and refugees bound for the United States: a population-based cross-sectional study. Ann Intern Med 2015; 162: 420–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Toms C, Stapledon R, Waring J, Douglas P, and the National Tuberculosis Advisory Committee. Tuberculosis notifications in Australia, 2012 and 2013. Commun Dis Intell Q Rep 2015; 39: E217–235. [PubMed] [Google Scholar]
- 22.Public Health England. UK pre-entry tuberculosis screening report 2015. London, UK: PHE, 2015. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/555150/UK_pre-entry_tuberculosis_screening_2015_GTW230916.pdf. Accessed April 2017. [Google Scholar]
- 23.Aldridge RW, Zenner D, White PJ, et al. Tuberculosis in migrants moving from high-incidence to low-incidence countries: a population-based cohort study of 519,955 migrants screened before entry to England, Wales, and Northern Ireland. Lancet 2016; 388: 2510–2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Institute of Environmental Science and Research Ltd. Tuberculosis in New Zealand: Annual Report 2014. Porirua, New Zealand: ESR, 2015. [Google Scholar]
- 25.Cain KP, Marano N, Kamene M, et al. The movement of multidrug-resistant tuberculosis across borders in East Africa needs a regional and global solution. PLOS Med 2015; 12: e1001791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moore BK, Posey DL, Maloney SA, Cetron M, Castro K. Tackling tuberculosis abroad: the key to TB elimination in the United States. A report CSIS Global Health Policy Center. Washington DC, USA: Center for Strategic and International Studies, 2014. [Google Scholar]
- 27.Schwartzman K, Oxlade O, Barr RG, et al. Domestic returns from investment in the control of tuberculosis in other countries. N Engl J Me 2005; 353: 10081020. [DOI] [PubMed] [Google Scholar]
- 28.United Nations, Department of Economic and Social Affairs, Population Division. International migration report 2015: highlights. New York, NY, USA: UN, 2016. [Google Scholar]
- 29.World Health Organization. The end TB strategy. Geneva, Switzerland: WHO, 2014. http://www.who.int/tb/strategy/End_TB_Strategy.pdf?ua=1. Accessed April 2017. [Google Scholar]
- 30.European Centre for Disease Prevention and Control. Multidrug-resistant tuberculosis in migrants, multi-country cluster. Stockholm, Sweden: ECDC, 2016. [Google Scholar]
- 31.Zimmerman C, Kiss L, Hossain M. Migration and health: a framework for 21st century policy-making. PLOS Med 2011: 8: e1001034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abarca Tomas B, Pell C, Bueno Cavanillas A, Guillen Solvas J, Pool R, Roura M. Tuberculosis in migrant populations. A systematic review of the qualitative literature. PLOS ONE 2013; 8: e82440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gushalak BD, MacPherson D W Health aspects of the pre-departure phase of migration. PLOS Med 2011; 8: e1001035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wickramage K, Mosca D. Can migration health assessments become a mechanism for global public health good? Int J Environ Res Public Health 2014; 11: 9954–9963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dhavan P, Mosca D. Tuberculosis and migration: a post 2015 call to action. Migration Policy Practice 2014; 4: 17–22. [Google Scholar]
- 36.Gushalak BD, MacPherson D W The basic principles of migration health: population mobility and gaps in disease prevalence. Emerg Themes Epidemiol 2006; 3: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Littleton J, Park J, Thornley C, Anderson A, Lawrence J. Migrants and tuberculosis: analysing epidemiological data with ethnography. Aust NZ J Public Health 2008; 32: 142–149. [DOI] [PubMed] [Google Scholar]
- 38.Falzon D, Zignol M, Migliori GB, Nunn P, Raviglione MC. Migration: an opportunity for the improved management of tuberculosis worldwide. Italian J Public Health 2012; 9: 1–11. [Google Scholar]
- 39.Zenner D, Southern J, van Hest R, et al. Active case finding for tuberculosis among high-risk groups in low-incidence countries. Int J Tuberc Lung Dis 2013; 17: 573–582. [DOI] [PubMed] [Google Scholar]
- 40.Uplekar M, Creswell J, Ottmani SE, Weil D, Sahu S, Lönnroth K. Programmatic approaches to screening for active tuberculosis. Int J Tuberc Lung Dis 2013; 17: 1248–1256. [DOI] [PubMed] [Google Scholar]
- 41.Aldridge RW, Yates TA, Zenner D, White PJ, Abubakar I, Hayward AC. Pre-entry screening programmes for tuberculosis in migrants to low-incidence countries: a systematic review and meta-analysis. Lancet Infect Dis 2014; 14: 1240–1249. [DOI] [PubMed] [Google Scholar]
- 42.Alvarez GG, Gushulak B, Abu Rumman K, et al. A comparative examination of tuberculosis immigration medical screening programs from selected countries with high immigration and low tuberculosis incidence rates. BMC Infect Dis 2011; 11: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.World Health Organization. Systematic screening for active tuberculosis: principles and recommendations. WHO/HTM/ TB/2013.04. Geneva, Switzerland: WHO, 2013. http://apps.who.int/iris/bitstream/10665/84971/1/9789241548601_eng.pdf?ua=1. Accessed April 2017. [PubMed] [Google Scholar]
- 44.Paglia M G Bevilacqua N, Haji HS, et al. Improvement of tuberculosis laboratory capacity on Pemba Island, Zanzibar: a health cooperation project. PLOS ONE 2012; 7: e44109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Atre S An urgent need for building technical capacity for rapid diagnosis of multidrug-resistant tuberculosis (MDR-TB) among new cases: a case report from Maharashtra, India. J Infect Public Health 2015; 8: 502–505. [DOI] [PubMed] [Google Scholar]
- 46.Plamondon KM, Hanson L, Labonte R, Abonyi S. The Global Fund and tuberculosis in Nicaragua: building sustainable capacity? Can J Public Health 2008; 99: 355–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Posey DL, Marano N, Cetron MS. Cross-border solutions needed to address tuberculosis in migrating populations. Int J Tuberc Lung Dis 2017; 21: 485–486. [DOI] [PubMed] [Google Scholar]
- 48.World Health Organization. A human rights approach to tuberculosis. WHO/CDS/STB/2001.9. Geneva, Switzerland: WHO, 2001. http://www.who.int/hhr/information/A%20Human%20Rights%20Approach%20to%20Tuberculosis.pdf. Accessed April 2017. [Google Scholar]


