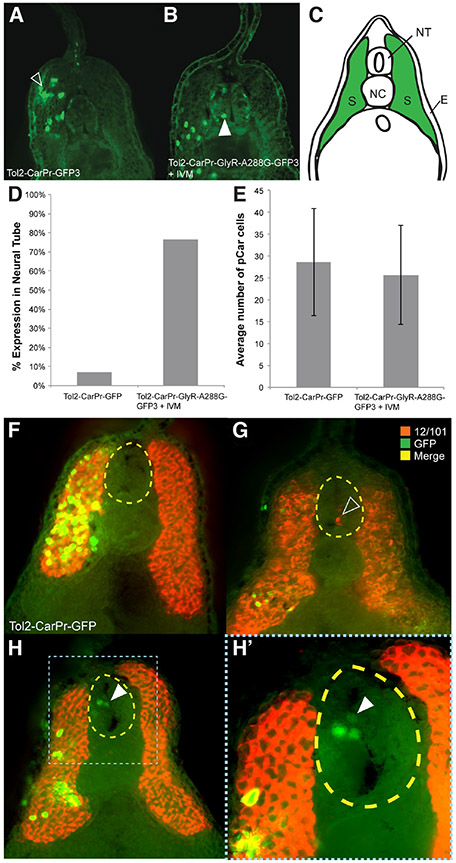
Fig. 4. Expressing GlyR-A288G and selectively depolarizing muscle-specific cells results in abnormal localization.
(A) Immunohistochemistry performed on sections of tailbud stage (NF stage 28/29) Xenopus laevis embryos that had been injected with Tol2 construct driving GFP expression in muscle cells using cardiac actin promoter, Car, using GFP-specific antibodies revealed normal muscle specific localization of GFP signal; in somatic tissue, green in (C). (B) Driving hypersensitive GlyR expression (GlyR-A288G) in muscle tissue using Car promoter and subsequent depolarization resulted in GFP signal appearing in neural tissue (white arrow). (D) Abnormal localization of GFP+-cells to the neural tube occurred in 76% of embryos that had been injected with Tol2-Car-pRGlyR-A288G-GFP3 and treated with 0.05 μM ivermectin, but overall number of GFP+ cells was not significantly different between injected and treated embryos and control embryos that had been injected with Tol2-CarPr-GFP3 (E). Co-immunohistochemistry using an anti-GFP antibody to track cells expressing GFP in response to the Car promoter and a 12/101 antibody labeling skeletal muscle confirmed GFP+ cells of Tol2-CarPr-GFP3 injected embryos are localized in somatic muscle tissue (F). Co-immunohistochemistry on embryos that had been injected with Tol2-CarPr-GlyR-A288G-GFP3 and treated with ivermectin displayed either 12/101 signal in the neural tube (G), or Car-regulated GFP signal in the neural tube [white arrow in (H, H’)] but no overlapping signal. Scale bars represent 100 μm.