Fig. 3. Images from optimal time points to check differentiation and characterization of the resulting skin organoid structure.
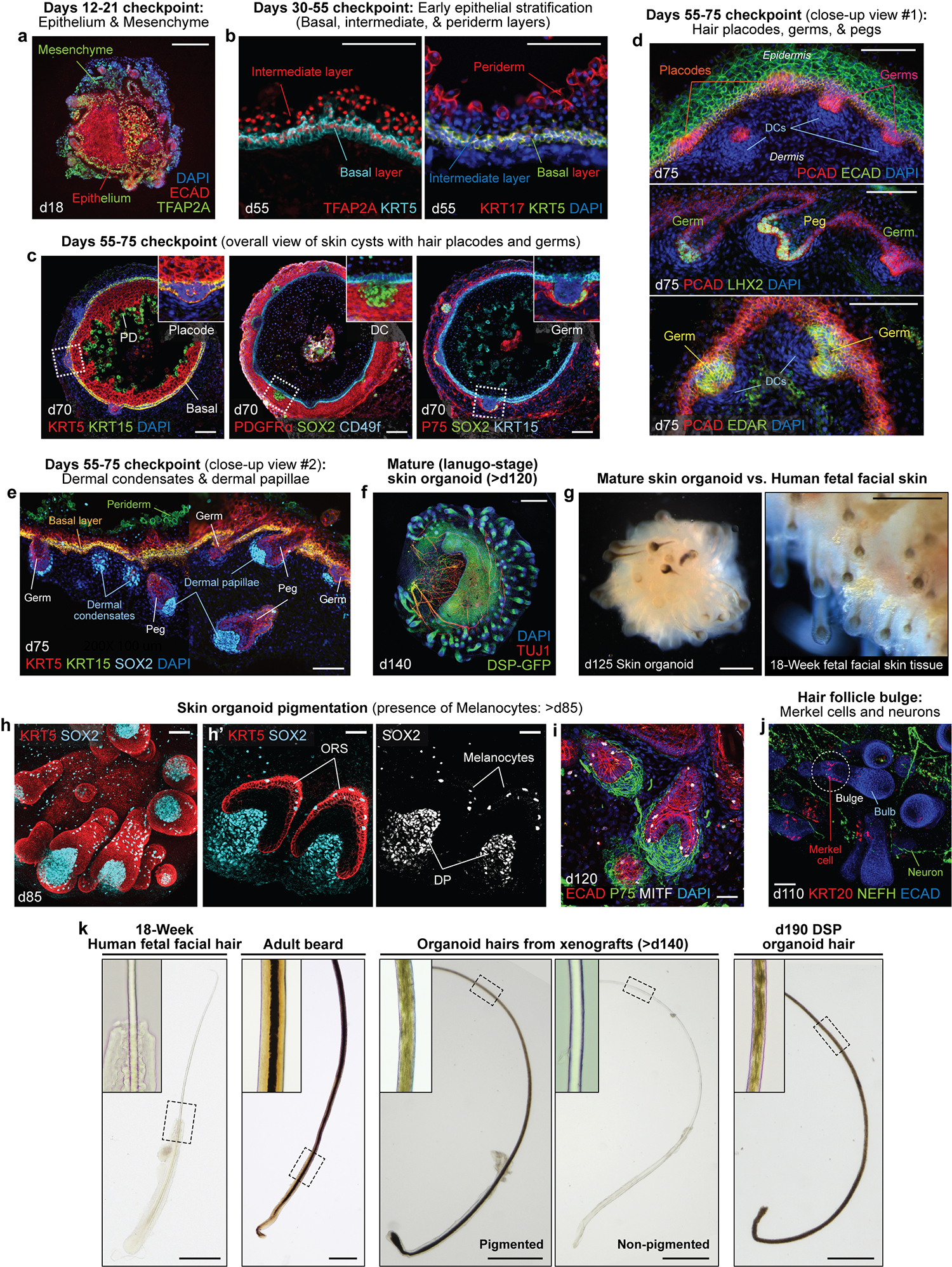
a-e, See Table 1 for further descriptions. Representative IHC images showing key checkpoints throughout the differentiation procedure of WA25 hESC-derived skin organoids. a, Day 18 image representing the ECAD+TFAP2A+ epithelium and TFAP2A+ CNC or mesenchymal cells surrounding the aggregate; the presence of these cell populations should be checked between days 6–20 of differentiation. b, Day 55 images showing TFAP2A+KRT5+ and KRT17+KRT5+ basal layer, TFAP2A+ intermediate layer, and KRT17+ periderm layer; the periderm layer is detectable only at early stages of differentiation, prior to formation of the granular and cornified layers; the periderm layer is visible in organoids around days 40–75 of differentiation. c, Overall-view image of day 70 skin cysts; the images show the major layers of skin that are required to form the skin, the epidermal and the dermal layers, and the initiating hair germs; the basal layer of skin is highlighted by KRT5+KRT15+ and CD49f+ fluorescence signals; the periderm layer is visualized by KRT15; the dermal layer (fibroblasts) is visualized by PDGFRα, and NC cell-derived mesenchymal cells within the population express P75; the SOX2+ cells represent dermal condensates at the tip of the hair germs; the initial hair placode and germ formations can be observed starting around day 55 of differentiation. PD, periderm; DC, dermal condensate. d,e, Day 75 high-magnification images representing PCAD+ hair placodes, PCAD+EDAR+LHX2+ hair germs, and PCAD+LHX2+ hair pegs; SOX2+ cells represent dermal condensates of hair germs and dermal papillae of hair pegs. f, A representative IHC image of a day 140 skin organoid. The endogenous green fluorescence from the DSP-GFP cell line visualizes epithelium of the skin cyst in the center and the hair follicles protruding from the surface of the cyst. TUJ1+ neurons are wrapping around and innervating the epithelium and the hair follicles. The skin organoids reach the lanugo-like mature stage around day 120 of differentiation. g, Representative darkfield images of a day 125 WA25 hESC-derived skin organoid (left) and dermal view of 18-week human fetal facial skin tissue (right). Skin organoids at days 120–140 resemble the mid-second trimester fetal skin tissue with (pigmented) hair follicles and adipocytes. h,i, Representative whole-mount immunostaining images of hair follicles with dermal papillae and melanocytes in a day 85 (h, left and middle) and a day 120 (i) WA25 hESC-derived skin organoids. KRT5 visualizes epithelium, outer root sheath (ORS) of hair follicles and newly forming hair germs (h, middle). SOX2 marks for melanocytes or Merkel cells present in the ORS of hair follicles and on the epithelium (h, right). MITF also specifies melanocytes in the ORS and on the epithelium. Dermal papillae of the hair follicles are also visualized by P75 (i). j, A representative whole-mount immunostaining image of a day 110 skin organoid hair follicles. The hair follicles contain a bulge region where KRT20+ touch-sensing Merkel cells are present. NEFH+ sensory neurons innervate the upper bulge region near Merkel cells. k, Representative brightfield images of plucked hairs from human fetal facial tissue at 18 weeks of gestation, adult male’s cheek (beard), skin organoid xenograft, and DSP skin organoid at day 190 of differentiation. Insets present a magnified area indicated with dash boxes. The medulla is only present in the adult beard. The medulla layer is not visible in xenograft hairs, either pigmented or non-pigmented. Darker hairs from a xenograft and a DSP skin organoid appear to contain pigmented cells that are scattered throughout the cortex, but no sign of medulla is detectable in the center of the hair shaft. See ref. 25 for additional images. The images are taken at the magnifications as follows: 200X (20X microscope objective × 10X eyepiece; b, d, e, h’); 100X (10X microscope objective × 10X eyepiece; a, c, h (left), i, and j); 50X (5X microscope objective × 10X eyepiece; g); 40X (4X microscope objective × 10X eyepiece; f and k (xenografts and organoid hairs)); 20X (2X microscope objective × 10X eyepiece; k (fetal hair and adult beard)). Scale bars, 500 μm (f, g, and k); 200 μm (a); 100 μm (b-e, and j); 50 μm (h (left) and i); 30 μm (h’).
