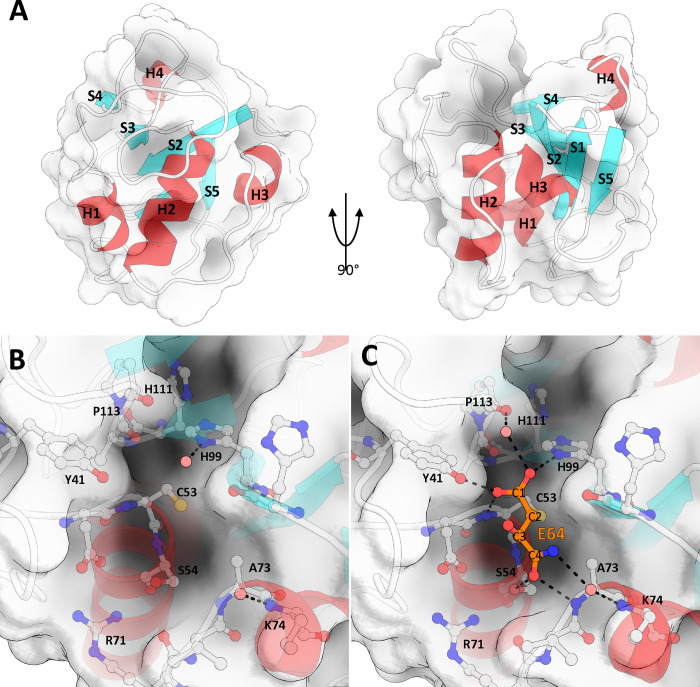
Fig 5. The structure of TvNlpC_B3.
(A) Papain-like fold of TvNlpC_B3, consisting of 3 N-terminal helices, and an anti-parallel beta-sheet structure, shown here at 0 and 90 degrees. A single helical turn, H4, is present between S4 and S5. The fold forms a binding cleft centered at the N-terminal beginning of helix H2 and running between strands S2 and S3, terminating on S4. (B) Active site and nearby residues of TvNlpC_B3, Cys53, His99, and His111 form a catalytic triad. (C) Active site when bound by E64 fragment, E64 covalently binds to TvNlpC_B3 active site via C2 and the thiol of Cys53.