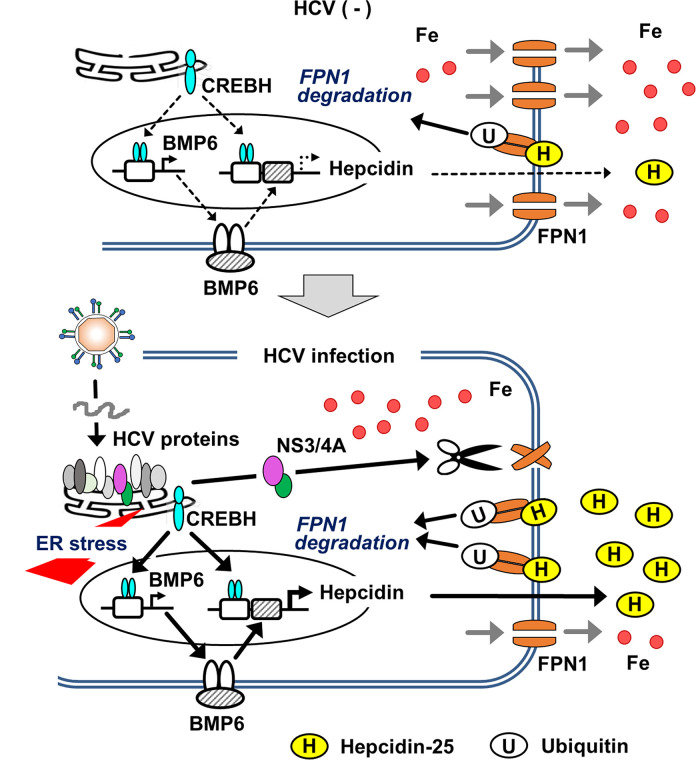
Fig 6. Working model of iron accumulation induced in HCV-infected hepatocytes.
Much of the replication process of HCV is dependent on the ER of the host cell; in HCV-infected cells, a viral precursor polyprotein is cleaved into 10 proteins by ER-resident peptidases and viral proteases associated with the ER membrane. ER-derived membranes also play an important role in viral replication complex formation. These characteristics of the HCV lifecycle induce ER stress in infected cells. This activates the ER resident transcription factor CREBH, which in turn upregulates the transcription both of BMP6 and hepcidin through its recognition of the promoters. The induction of BMP6 expression enhances BMP signaling, and the Smad complex, which is activated as a result, also plays a role in activation of the hepcidin promoter. The induction of hepcidin gene expression leads to an increase in extracellular hepcidin peptide levels, which in turn increases hepcidin-FPN1 association, thus promoting retrograde transport of FPN1 into the cell and ubiquitin-dependent degradation. The NS3-NS4A complex, whose serine protease activity is essential for processing the viral precursor, can cleave the intracytoplasmic portion of the central region of FPN1, which is presumably suppressed in its iron efflux function upon cleavage.