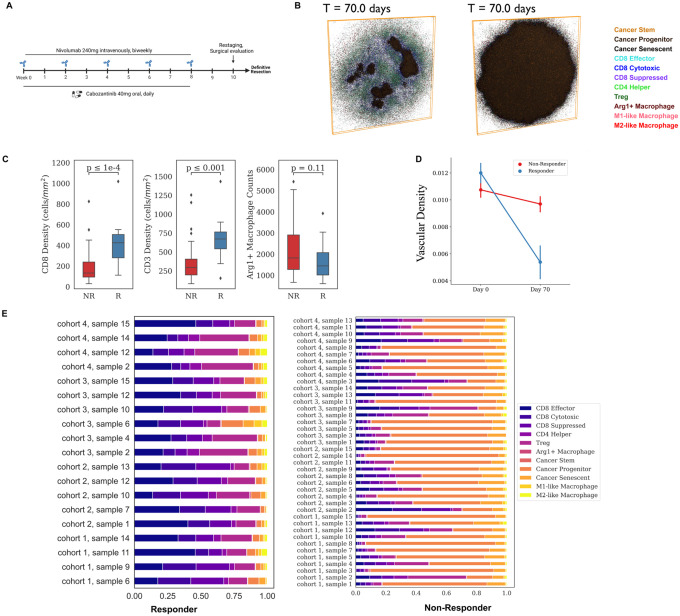
Fig 3. Results for the virtual clinical trial.
A) Dosing strategy of nivolumab and cabozantinib in both the phase 1b HCC neoadjuvant clinical trial and spQSP virtual clinical trial simulations. Nivolumab (240mg) is injected intravenously every 2 weeks for 8 weeks. Cabozantinib is administered orally every day for 8 weeks. B) Two-dimensional cross section of the spatial distribution of cells in the tumor compartment from a representative simulation at day 70 for both responders and non-responders. Simulation movies for three-dimensional cellular states over time are provided in Supplement Movies. C) Quantitative comparison of CD8+, CD3+, and Arg1+ Macrophage in the stratified patient groups (responder: n=19 vs. non-responder: n=40) at day 70. D) Longitudinal dynamics of average vascular density in the ABM sample of two groups of patients (R vs. NR). E) Cell composition in the ABM model outputs at day 70, grouped by treatment outcomes.